
Assertion:
The second substituent may enter the mono-substituted benzene ring at either ortho, para or meta position.
Reason:
The position of the incoming group is determined by the nature of the group present in the monosubstituted benzene ring.
A. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B. Both Assertion and Reason are correct and Reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is correct.
D. Both assertion and reason are incorrect.
Answer
592.2k+ views
Hint: There are two types of groups which are found to be attached with the benzene ring. It may be an Electron Withdrawing group or Electron Donating group because both shows have a different effect on the upcoming group or second substituent.
Complete step by step answer:
- In the given assertion and given both are the correct statements.
- Because there are two types of the substituent which attach on the benzene ring and show their affection.
- The position on which they are attached also plays a key role because it influences the ring.
- There are three-position mentioned in the aromatic ring i.e. ortho, para and meta position.
- Ortho position is present adjacent to the functional group whereas meta position is the position which is adjacent to the ortho position and para position is adjacent to the meta position.
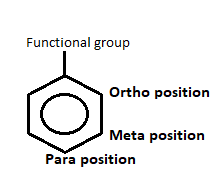
- Here the functional group is of two types either the electron-withdrawing group or electron-donating group.
- Electron withdrawing groups are those species in which the electron density is withdrawn by the neighbouring elements.
For example, -CN, -COOH, etc.
- The electron-withdrawing group has only one major group that is meta position and causes the second substituent to attach to the meta position.
-Whereas the electron-donating groups are those species which have a lone pair of an electron to donate to the neighbouring atoms.
For example, the alkyl group.
- The electron-donating group has two major groups that are ortho and para position and causes the second substituent to attach on either ortho or para position.
- So, we can say that the nature of the second substituent depends on the nature of the mon-substituent group.
Therefore, Option A is the correct answer because both the statements are correct and the reason is the correct explanation of the assertion.
Note: The mono-substituent benzene ring is formed by the process of Electrophilic Aromatic Substitution. The addition of the second substituent is also dependent on the resonance (delocalisation of pi- bond) and inductive (an electronic effect caused by the polarisation of sigma bond) effects.
Complete step by step answer:
- In the given assertion and given both are the correct statements.
- Because there are two types of the substituent which attach on the benzene ring and show their affection.
- The position on which they are attached also plays a key role because it influences the ring.
- There are three-position mentioned in the aromatic ring i.e. ortho, para and meta position.
- Ortho position is present adjacent to the functional group whereas meta position is the position which is adjacent to the ortho position and para position is adjacent to the meta position.

- Here the functional group is of two types either the electron-withdrawing group or electron-donating group.
- Electron withdrawing groups are those species in which the electron density is withdrawn by the neighbouring elements.
For example, -CN, -COOH, etc.
- The electron-withdrawing group has only one major group that is meta position and causes the second substituent to attach to the meta position.
-Whereas the electron-donating groups are those species which have a lone pair of an electron to donate to the neighbouring atoms.
For example, the alkyl group.
- The electron-donating group has two major groups that are ortho and para position and causes the second substituent to attach on either ortho or para position.
- So, we can say that the nature of the second substituent depends on the nature of the mon-substituent group.
Therefore, Option A is the correct answer because both the statements are correct and the reason is the correct explanation of the assertion.
Note: The mono-substituent benzene ring is formed by the process of Electrophilic Aromatic Substitution. The addition of the second substituent is also dependent on the resonance (delocalisation of pi- bond) and inductive (an electronic effect caused by the polarisation of sigma bond) effects.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




