
Assertion
\[S{O_3}\] has a planar structure.
Reason
Sulphur atom in \[S{O_3}\] is \[S{p^2}\] hybridized and \[O - S - O\] bond angle in \[120^\circ \]
A.If both assertion and reason are CORRECT and reason is the CORRECT explanation of the assertion.
B.If both assertion and reason are CORRECT, but reason is NOT THE CORRECT explanation of the assertion.
C.If the assertion is CORRECT, but the reason is INCORRECT.
D.If assertion is INCORRECT, but reason is CORRECT.
Answer
559.2k+ views
Hint:We must have to know that the chemical compound with the formula $SO_3$ is sulphur trioxide, with a relatively small liquid range. This species is a major pollutant in its gaseous form, being the primary precursor to acid rain. As a precursor to sulfuric acid, it is prepared on an industrial scale and is also known as sulfuric anhydride.
As we know that the shape of a molecule depends on the atoms which make up it and the electrons belonging to the central atom. The molecule is planar if the atoms organize themselves around the central molecule such that they reside on a single two-dimensional plane.
Complete step by step answer:
We know that the trigonal planar is a molecular geometry model of chemistry with one atom at the centre and three atoms at the corners of an equilateral triangle, referred to as peripheral atoms, all in one plane. All three ligands are equal in an ideal trigonal planar species and all bond angles are \[120^\circ \].
Sulfur trioxide is known to be a sulfuric acid anhydride. The trigonal planar structure of \[S{O_3}\] has a bond angle of \[120^\circ \]. The \[S{O_3}\] molecule representation is shown below. The triangular planar molecule \[S{O_3}\] in the gas phase requires sp2 hybridization of the S atom. There are three sigma bonds formed by overlap of \[s{p^2} - p\] and three pi bonds, one by overlap of p(π)-p(π) and two by overlap of p(π)-d(π). The bond angles of \[O - S - O\] are \[120^\circ \].
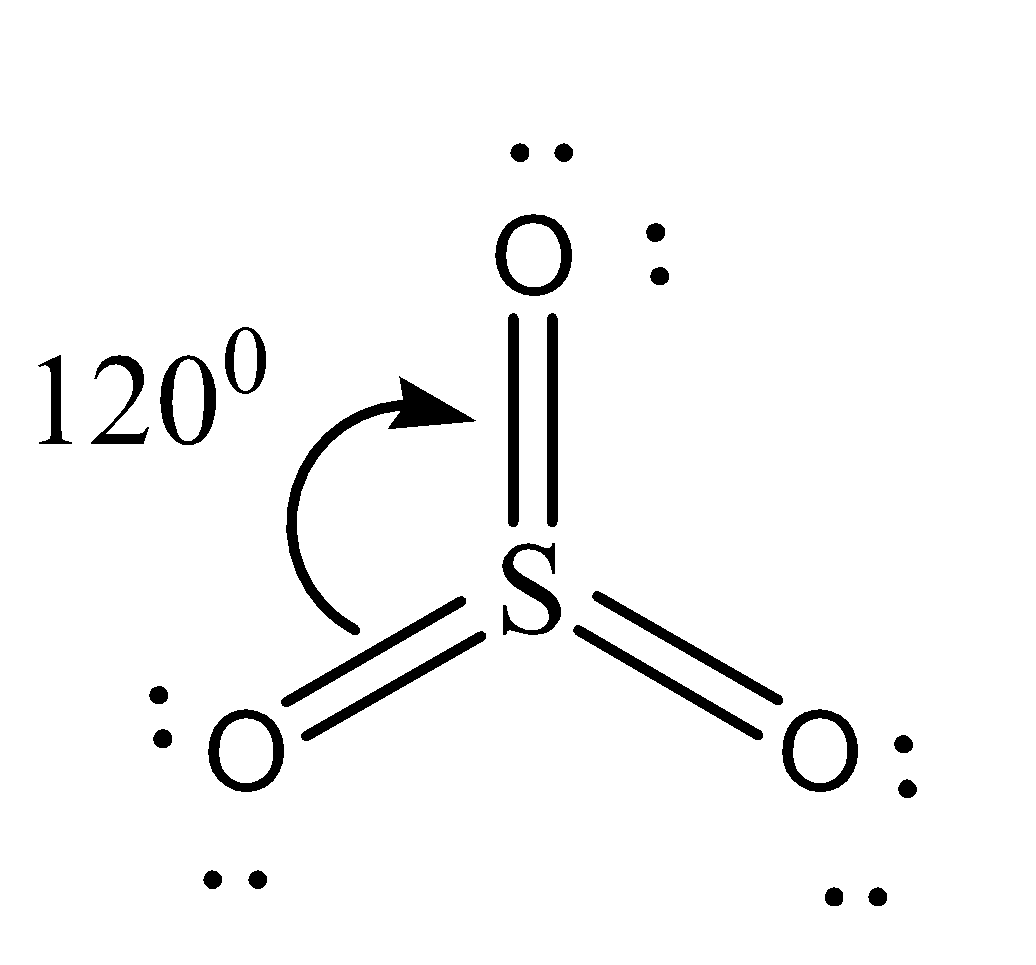
Option C is correct as the reason given is incorrect and the assertion is correct.
Note:
We must have to remember that the hybridization of \[s{p^2}\] is the combining of atomic orbitals of one s and two p, which includes the promotion of one electron to one of the atomic orbitals of 2p in the s orbital. Three new hybrid orbitals equal to the energy level are formed by the combination of these atomic orbitals.
Pi bonds are covalent chemical bonds where two orbital lobes on one atom overlap with two orbital lobes on another atom, and this overlap takes place laterally. Each of these atomic orbitals, moving through the two bonded nuclei, has zero electron density in a shared nodal plane.
As we know that the shape of a molecule depends on the atoms which make up it and the electrons belonging to the central atom. The molecule is planar if the atoms organize themselves around the central molecule such that they reside on a single two-dimensional plane.
Complete step by step answer:
We know that the trigonal planar is a molecular geometry model of chemistry with one atom at the centre and three atoms at the corners of an equilateral triangle, referred to as peripheral atoms, all in one plane. All three ligands are equal in an ideal trigonal planar species and all bond angles are \[120^\circ \].
Sulfur trioxide is known to be a sulfuric acid anhydride. The trigonal planar structure of \[S{O_3}\] has a bond angle of \[120^\circ \]. The \[S{O_3}\] molecule representation is shown below. The triangular planar molecule \[S{O_3}\] in the gas phase requires sp2 hybridization of the S atom. There are three sigma bonds formed by overlap of \[s{p^2} - p\] and three pi bonds, one by overlap of p(π)-p(π) and two by overlap of p(π)-d(π). The bond angles of \[O - S - O\] are \[120^\circ \].

Option C is correct as the reason given is incorrect and the assertion is correct.
Note:
We must have to remember that the hybridization of \[s{p^2}\] is the combining of atomic orbitals of one s and two p, which includes the promotion of one electron to one of the atomic orbitals of 2p in the s orbital. Three new hybrid orbitals equal to the energy level are formed by the combination of these atomic orbitals.
Pi bonds are covalent chemical bonds where two orbital lobes on one atom overlap with two orbital lobes on another atom, and this overlap takes place laterally. Each of these atomic orbitals, moving through the two bonded nuclei, has zero electron density in a shared nodal plane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




