
Assertion: Lactic acid is optically active
Reason:A symmetry in the inner structure of organic compounds causes optical activity.
A.Both assertion and reason are correct and the reason is the correct explanation of the assertion.
B.Both assertion and reason are correct but reason is not the correct explanation of the assertion.
C.Assertion is correct but reason is incorrect.
D.Both reason and assertion is incorrect.
Answer
498.3k+ views
Hint: To solve this question first we need to understand the concept that defines the ‘optical activity’ of the particular compound. So, for the compound or organic compound to be optically active, it should have the ‘chiral centre or chiral carbon’.
Complete answer:
So as we know that for the compound to be optically active it should be chiral. So for the compound to be chiral, it should have chiral centre or chiral carbon.
Now let’s look at what the term; chirality or chiral carbon means.
So, for a better understanding of the question, let’s have a look at the structure of the lactic acid.
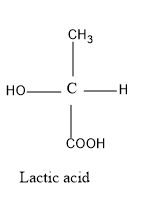
Thus as from the above figure we can see that in lactic acid, the carbon atom contains four different functional groups i.e. methyl group, carboxyl , hydrogen and the hydroxyl group.
Thus we can say that lactic acid has a chiral carbon and is optically active, so, our assertion is correct i.e. lactic acid is optically active.
Now when we look at the structure of lactic acid, we see the asymmetry in the structure and not a symmetry.
So, the reason is wrong in itself and is also not the correct explanation of the assertion.
Thus from the above discussion, we can say that the correct answer is option C i.e. Assertion is correct but the reason is incorrect.
Option C is the correct answer.
Note:
So, the chiral centre or chiral carbon is the atom or the carbon, in the compound which has four different functional groups attached to it i.e. the valence of the carbon must be satisfied by four different functional groups.
Complete answer:
So as we know that for the compound to be optically active it should be chiral. So for the compound to be chiral, it should have chiral centre or chiral carbon.
Now let’s look at what the term; chirality or chiral carbon means.
So, for a better understanding of the question, let’s have a look at the structure of the lactic acid.

Thus as from the above figure we can see that in lactic acid, the carbon atom contains four different functional groups i.e. methyl group, carboxyl , hydrogen and the hydroxyl group.
Thus we can say that lactic acid has a chiral carbon and is optically active, so, our assertion is correct i.e. lactic acid is optically active.
Now when we look at the structure of lactic acid, we see the asymmetry in the structure and not a symmetry.
So, the reason is wrong in itself and is also not the correct explanation of the assertion.
Thus from the above discussion, we can say that the correct answer is option C i.e. Assertion is correct but the reason is incorrect.
Option C is the correct answer.
Note:
So, the chiral centre or chiral carbon is the atom or the carbon, in the compound which has four different functional groups attached to it i.e. the valence of the carbon must be satisfied by four different functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




