
Assertion: Coordination number of both $N{{a}^{+}}$ and $C{{l}^{-}}$ in NaCl is 6.
Reason: Second coordination number of $C{{l}^{-}}$ in the NaCl unit is 12.
Answer
538.5k+ views
Hint: The structure of the crystal can be calculated by seeing the radius ratio of the cations and the anions. Now, to calculate the coordination number, count the number of opposite ions present, and to calculate the second coordination number see the nearest number of the same ions in the crystal.
Complete step by step solution: NaCl is a solid crystal and we can calculate the radius ratio of the ions. The ratio of the radius of the $N{{a}^{+}}$ to the radius of $C{{l}^{-}}$ ions comes between 0.414 – 0.732, so it means that the crystal is FCC in which the chloride ions are on the face-centers and corner of the crystal and the sodium ion is over the octahedral voids.
Since the radius ratio of the NaCl is between 0.414 – 0.732, therefore, the structural arrangement is octahedral so, the coordination number of the ions is 6. This means that the number of $C{{l}^{-}}$ ions surrounding the $N{{a}^{+}}$ are 6 and vice-versa. Therefore, the assertion is correct.
Now, to calculate the second coordination number of chloride ions, we have to calculate the number of chloride ions surrounding all three axes. We know that the chloride ions are present on the face centers and corners of the crystal. When one atom is present on the corner of the crystal, then the nearest same atom will be at the center of the face as shown below:
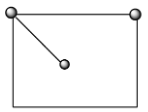
So, one atom in the corner is attached with 4 same atoms on one axis, therefore, in all the three-axis there are 12 atoms surrounding. Hence, the second coordination number of $C{{l}^{-}}$ ion is 12.
Therefore, the assertion is correct. The reason is also correct but it is not the correct explanation for the assertion.
Note: If the radius ratio is between 0.155 – 0.225, then the first coordination number is taken 3, if the radius ratio is between 0.225 – 0.414 then the first coordination number is taken 4, and the radius ratio is between 0.732 - 1 then the first coordination number is taken 8.
Complete step by step solution: NaCl is a solid crystal and we can calculate the radius ratio of the ions. The ratio of the radius of the $N{{a}^{+}}$ to the radius of $C{{l}^{-}}$ ions comes between 0.414 – 0.732, so it means that the crystal is FCC in which the chloride ions are on the face-centers and corner of the crystal and the sodium ion is over the octahedral voids.
Since the radius ratio of the NaCl is between 0.414 – 0.732, therefore, the structural arrangement is octahedral so, the coordination number of the ions is 6. This means that the number of $C{{l}^{-}}$ ions surrounding the $N{{a}^{+}}$ are 6 and vice-versa. Therefore, the assertion is correct.
Now, to calculate the second coordination number of chloride ions, we have to calculate the number of chloride ions surrounding all three axes. We know that the chloride ions are present on the face centers and corners of the crystal. When one atom is present on the corner of the crystal, then the nearest same atom will be at the center of the face as shown below:

So, one atom in the corner is attached with 4 same atoms on one axis, therefore, in all the three-axis there are 12 atoms surrounding. Hence, the second coordination number of $C{{l}^{-}}$ ion is 12.
Therefore, the assertion is correct. The reason is also correct but it is not the correct explanation for the assertion.
Note: If the radius ratio is between 0.155 – 0.225, then the first coordination number is taken 3, if the radius ratio is between 0.225 – 0.414 then the first coordination number is taken 4, and the radius ratio is between 0.732 - 1 then the first coordination number is taken 8.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




