
Assertion
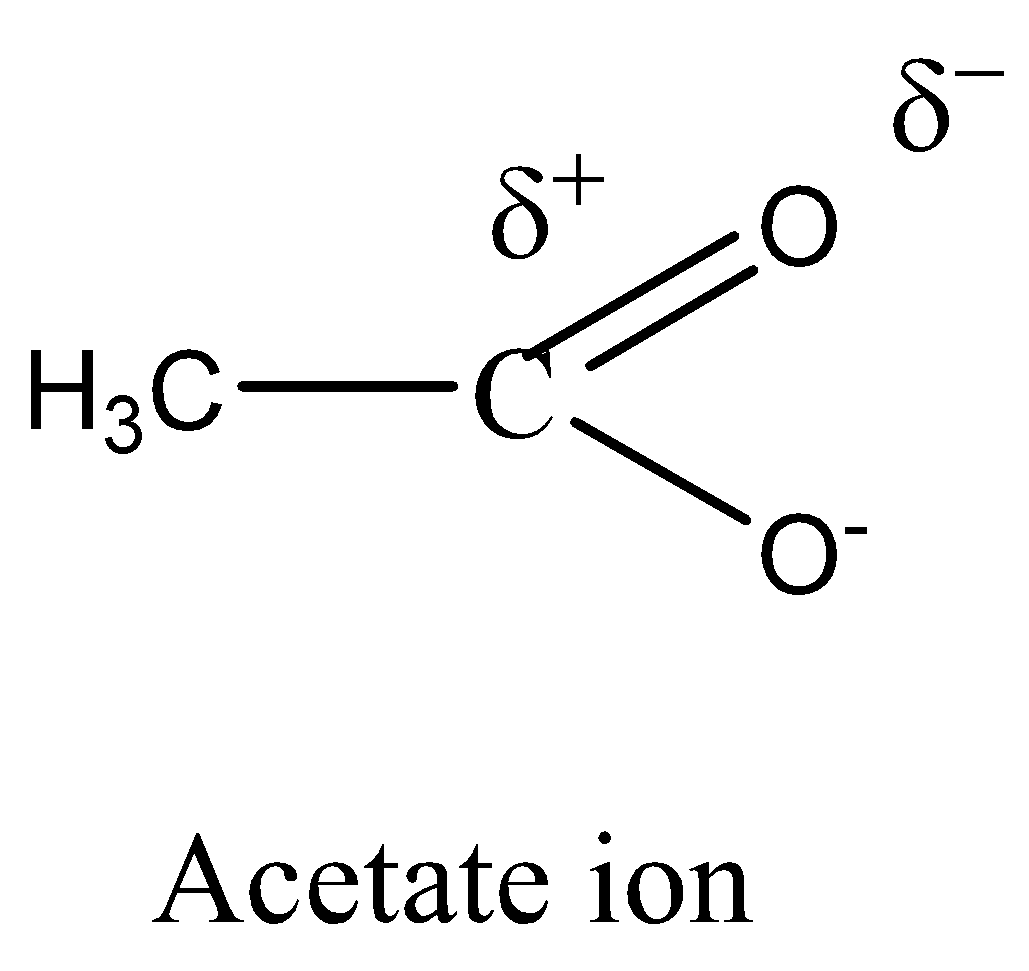
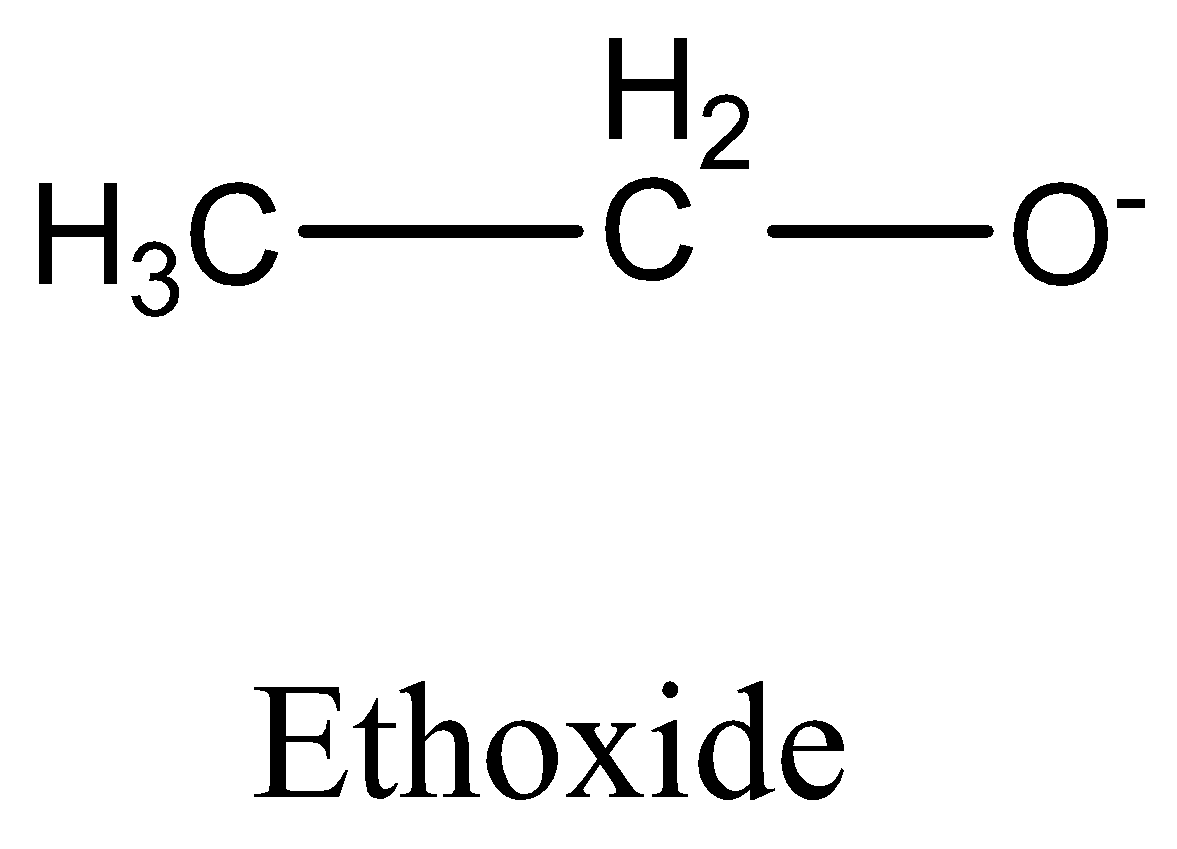
\[C{H_3}CO{O^ - }\] (acetate ion) is more stable than \[{C_2}{H_5}{O^ - }\] (ethoxide ion).
Reason
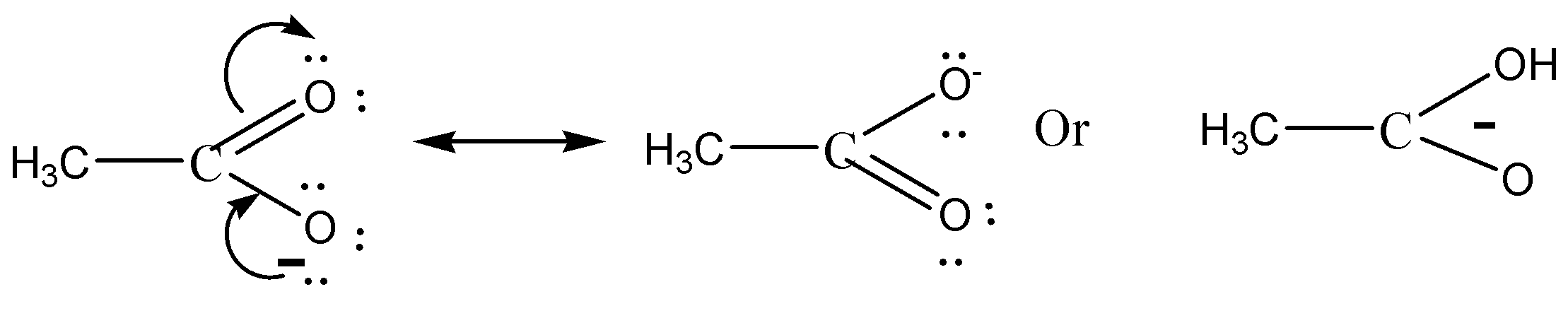
Electron delocalisation causes the negative charge in acetate to be shared equally by both oxygens. This type of resonance effect is not possible in ethoxide ion.
A.Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
B.Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
C.Assertion is correct but Reason is incorrect
D.Both Assertion and Reason are incorrect
Answer
559.2k+ views
Hint:We need to remember that acetate is an anion of monocarboxylic acid that formed from acetic acid carboxyl group by the removal of a proton. As a human metabolite and a metabolite of Saccharomyces cerevisiae, it has a function. It is an acetic acid conjugate base.
The ethoxide ion is a nucleophile that is almost "bare" It can attack the substrate better and is therefore a stronger nucleophile. The ethoxide ion is also a strong base, so the aldol condensation is a competing reaction in acetone.
Complete step by step answer:
We must need to remember that the \[C{H_3}CO{O^ - }\] is more stable than \[{C_2}{H_5}{O^ - }\] (acetate ion) (ethoxide ion) respectively. It is due to the delocalization of electrons. Resonance structures induce all oxygens to share the negative charge in acetate equally. As the +I inductive effect of \[{C_2}{H_5}{O^ - }\] makes it unstable, this resonance effect is not possible in ethoxide ions.
Both assertion and justification are also valid and reason is the right explanation for assertion.
In chemistry, the inductive effect is an effect on the transmission across a chain of atoms in a molecule of the unequal sharing of the bonding electron, leading to a permanent dipole in a bond.
Option A is the correct.
Note:
As we know that in chemistry, delocalized electrons are electrons which are not connected with a single atom or a covalent bond in a molecule, ion or solid metal. The word delocalization is general and in various fields it can have slightly different definitions. The resonance structure of a molecule depends on interaction between sigma and pi bond or two adjacent atoms.
The ethoxide ion is a nucleophile that is almost "bare" It can attack the substrate better and is therefore a stronger nucleophile. The ethoxide ion is also a strong base, so the aldol condensation is a competing reaction in acetone.
Complete step by step answer:
We must need to remember that the \[C{H_3}CO{O^ - }\] is more stable than \[{C_2}{H_5}{O^ - }\] (acetate ion) (ethoxide ion) respectively. It is due to the delocalization of electrons. Resonance structures induce all oxygens to share the negative charge in acetate equally. As the +I inductive effect of \[{C_2}{H_5}{O^ - }\] makes it unstable, this resonance effect is not possible in ethoxide ions.



Both assertion and justification are also valid and reason is the right explanation for assertion.
In chemistry, the inductive effect is an effect on the transmission across a chain of atoms in a molecule of the unequal sharing of the bonding electron, leading to a permanent dipole in a bond.
Option A is the correct.
Note:
As we know that in chemistry, delocalized electrons are electrons which are not connected with a single atom or a covalent bond in a molecule, ion or solid metal. The word delocalization is general and in various fields it can have slightly different definitions. The resonance structure of a molecule depends on interaction between sigma and pi bond or two adjacent atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




