
Assertion: All the S atoms in ${S_4}{O_6}^{2 - }$ have oxidation number equal to $ + 2.5$.
Reason: Average oxidation number of sulphur in ${S_4}{O_6}^{2 - }$ is $ + 2.5$.
A.Both assertion and reason are correct and the reason is the correct explanation for assertion.
B.Both assertion and reason are correct but reason is not the correct explanation for assertion.
C.Assertion is correct but the reason is incorrect.
D.Assertion is incorrect but the reason is correct.
Answer
571.5k+ views
Hint: We can define oxidation state as oxidation degree (loss of an electron) in a chemical compound. We can calculate the oxidation state of an element in a compound by using the rules of oxidation numbers. We have to calculate the oxidation state of sulfur in ${S_4}{O_6}^{2 - }$ using the number of oxygen atoms, charge of the compound and oxidation state of oxygen.
Complete step by step solution:
We have to remember that the oxidation state could be described as oxidation degree (electron loss) in a chemical compound. We know that $ - 2$ is the oxidation state of oxygen. The total of the oxidation number is equal to the charge is $ - 2$.
We can draw the structure of ${S_4}{O_6}^{2 - }$ as,
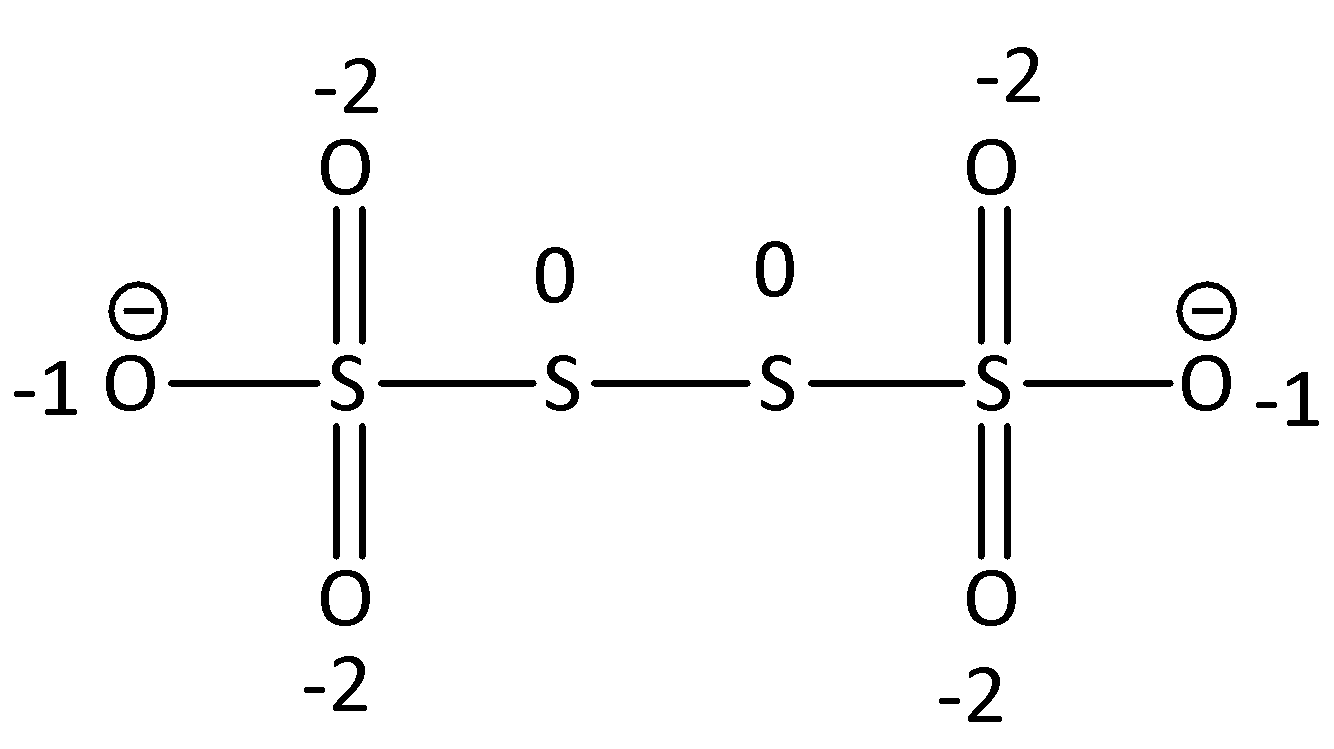
In the middle, two sulphurs contain zero oxidation state as an atom that is attached with the same atom that has an oxidation state of zero. Then, the oxidation state of the leftmost and rightmost sulphur is +5 since the oxygen is more electronegative.
So, the oxidation state of sulphur is $n - 2 - 2 - 1 + 0 = 0$
$n = 5$
Therefore, the oxidation state of sulfur is $ + 5$ isolated $S - S$ linkage contains zero oxidation state.
Let x be the oxidation number of sulfur.
$4x + 6\left( { - 2} \right) = - 2$
On multiplying we get,
$ \Rightarrow 4x - 12 = - 2$
$4x = 10$
On simplifying we get,
$ \Rightarrow x = 2.5$
From the above explanations, we can see that the average oxidation state of sulfur is $ + 2.5$.
In ${S_4}{O_6}^{2 - }$ all atoms of sulfur do not have an oxidation number equal to $ + 2.5$.
The oxidation state of sulfur is $ + 5,0,0, + 5$.
So, the assertion is not correct but the reason is correct.
Therefore, the option (D) is correct.
Note:We have to remember that in oxoacids, the structure of sulfur is tetrahedral with respect to oxygen. Oxygen atoms are present in oxoacids. The oxoacids contain a minimum of one $S = O$ bond and one bond of $S - OH$. In oxoacids, there are also terminal peroxide groups, one $S = S$ terminal group, terminal and bridging atoms of oxygen.
Complete step by step solution:
We have to remember that the oxidation state could be described as oxidation degree (electron loss) in a chemical compound. We know that $ - 2$ is the oxidation state of oxygen. The total of the oxidation number is equal to the charge is $ - 2$.
We can draw the structure of ${S_4}{O_6}^{2 - }$ as,

In the middle, two sulphurs contain zero oxidation state as an atom that is attached with the same atom that has an oxidation state of zero. Then, the oxidation state of the leftmost and rightmost sulphur is +5 since the oxygen is more electronegative.
So, the oxidation state of sulphur is $n - 2 - 2 - 1 + 0 = 0$
$n = 5$
Therefore, the oxidation state of sulfur is $ + 5$ isolated $S - S$ linkage contains zero oxidation state.
Let x be the oxidation number of sulfur.
$4x + 6\left( { - 2} \right) = - 2$
On multiplying we get,
$ \Rightarrow 4x - 12 = - 2$
$4x = 10$
On simplifying we get,
$ \Rightarrow x = 2.5$
From the above explanations, we can see that the average oxidation state of sulfur is $ + 2.5$.
In ${S_4}{O_6}^{2 - }$ all atoms of sulfur do not have an oxidation number equal to $ + 2.5$.
The oxidation state of sulfur is $ + 5,0,0, + 5$.
So, the assertion is not correct but the reason is correct.
Therefore, the option (D) is correct.
Note:We have to remember that in oxoacids, the structure of sulfur is tetrahedral with respect to oxygen. Oxygen atoms are present in oxoacids. The oxoacids contain a minimum of one $S = O$ bond and one bond of $S - OH$. In oxoacids, there are also terminal peroxide groups, one $S = S$ terminal group, terminal and bridging atoms of oxygen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




