
Assertion (A): \[HP{O_3}\] is a monobasic acid
Reason (R): The salts of \[HP{O_3}\]are called Meta phosphates
A.Both (A) and (R) are true and (R) is the correct explanation of (A)
B.Both (A) and (R) are true and (R) is not a correct explanation of (A)
C.(A) is true and (R) is false
D.(A) is false but (R) is true
Answer
519.3k+ views
Hint: We have to remember that the \[HP{O_3}\] is an organic compound and its chemical name is metaphosphoric acid. It is coming under the group of phosphoric acid and it dissolves slowly in cold water which decomposes into phosphoric acid. And it is also known as hydrogen phosphonate. And metaphosphoric acid is a monobasic acid which will stabilize the ascorbic acid.
Complete answer:
We must have to know that the metaphosphoric acid is a monobasic compound and the salts of \[HP{O_3}\] are called Meta phosphates. But the reason is not a correct explanation for the assertion. Hence, the option (A) is incorrect.
As we know that \[HP{O_3}\] is an organic compound and its chemical name is metaphosphoric acid. It is a monobasic compound. Because it contains only one hydrogen ion or one replaceable hydrogen atom per one molecule in water. The structure of \[HP{O_3}\]can be drawn as,
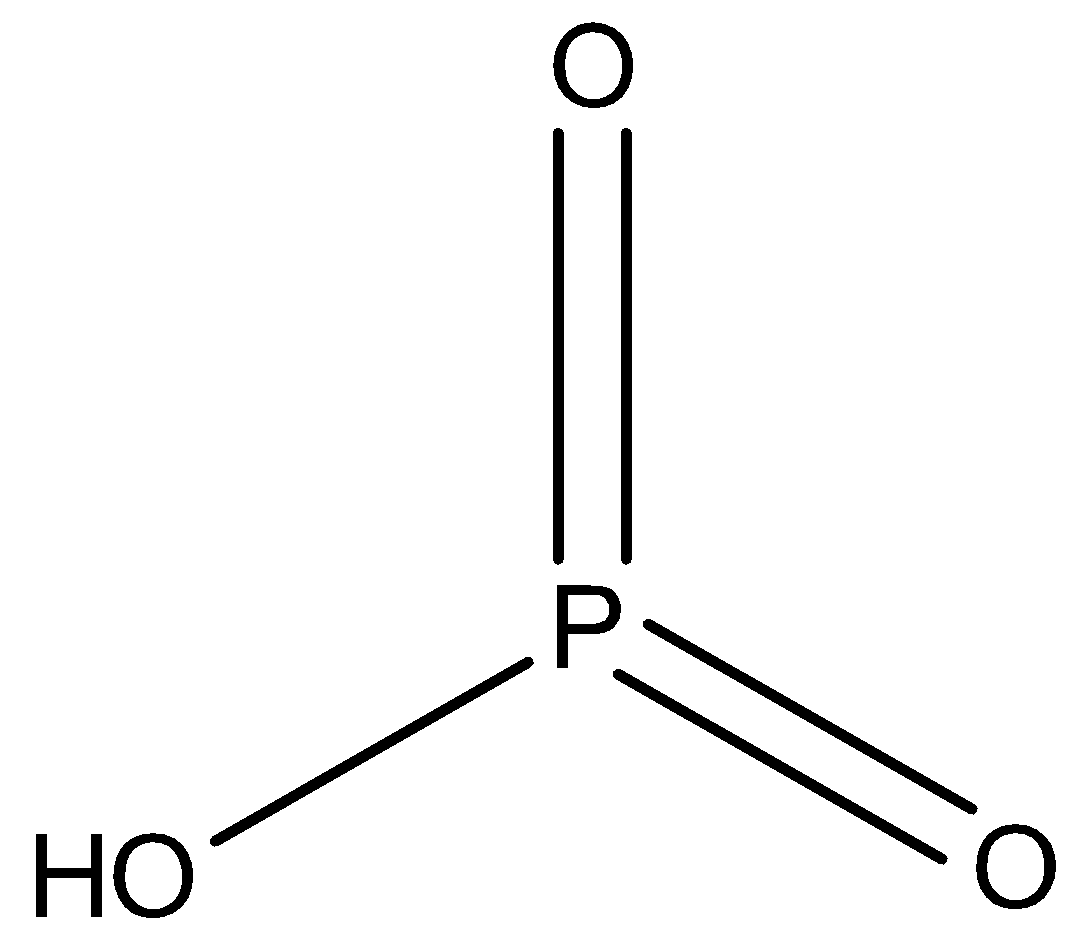
And the salts of \[HP{O_3}\] are called Meta phosphates. But this is not a correct explanation for the assertion.
Hence, the option (B) is correct.
In these statements, both assertion and reason are true. Hence, the option (C) is incorrect.
Here, both assertion and reason are correct. The reason is not a correct explanation for assertion. Hence, the option (D) is incorrect.
Note:
We must have to remember that the \[HP{O_3}\] is an organic compound and its chemical name is metaphosphoric acid which is mainly used to prepare ascorbic acid and to eliminate copper, which is also used to measure the amount of vitamin C. It is a monobasic compound. And the salts of \[HP{O_3}\] are called Meta phosphates. But the salts of metaphosphoric acid is not a reason for its acidic property.
Complete answer:
We must have to know that the metaphosphoric acid is a monobasic compound and the salts of \[HP{O_3}\] are called Meta phosphates. But the reason is not a correct explanation for the assertion. Hence, the option (A) is incorrect.
As we know that \[HP{O_3}\] is an organic compound and its chemical name is metaphosphoric acid. It is a monobasic compound. Because it contains only one hydrogen ion or one replaceable hydrogen atom per one molecule in water. The structure of \[HP{O_3}\]can be drawn as,

And the salts of \[HP{O_3}\] are called Meta phosphates. But this is not a correct explanation for the assertion.
Hence, the option (B) is correct.
In these statements, both assertion and reason are true. Hence, the option (C) is incorrect.
Here, both assertion and reason are correct. The reason is not a correct explanation for assertion. Hence, the option (D) is incorrect.
Note:
We must have to remember that the \[HP{O_3}\] is an organic compound and its chemical name is metaphosphoric acid which is mainly used to prepare ascorbic acid and to eliminate copper, which is also used to measure the amount of vitamin C. It is a monobasic compound. And the salts of \[HP{O_3}\] are called Meta phosphates. But the salts of metaphosphoric acid is not a reason for its acidic property.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




