
Assertion (A): Cellulose and starch cannot form the osazone
Reason (R): The monomer of the cellulose is $\beta - D - $ glucose and the monomer of starch is $\alpha - D - $ Glucose.
A.A and R are true and R is the correct explanation of A.
B.A and R are true and R is not the correct explanation of A.
C.A is true but R is false
D.A is false but R is true
Answer
581.4k+ views
Hint: Basically, cellulose and starch are identical polymers that have the same units which are further dependent on glucose and are composed of the same glucose and monomers. Further monomer refers to the simple molecule with two or more binding sites through which it forms covalent linkages with other monomer molecules to form the macromolecule.
Complete step by step answer:
Cellulose is a complex carbohydrate with chemical formula ${({C_6}{H_{10}}{O_5})_n}$ . It is odorless and insoluble in water and most organic solvents. On the other hand, starch is weaker than cellulose and can be dissolved in warm water. Further, cellulose is not suitable for human consumption.
Now, osazones are a class of carbohydrates derivatives found in organic chemistry which are formed when reducing sugars are reacted with excess phenylhydrazine at boiling temperatures. This reaction was developed by famous German chemist Emil Fischer. Now, cellulose and starch cannot form osazone. This is because the aldehyde group is not free and it is involved in the formation of glycosidic linkage. Moreover, the monomer of cellulose is D-Glucose and the monomer of Starch is D-glucose. The structure of glucose is as shown:
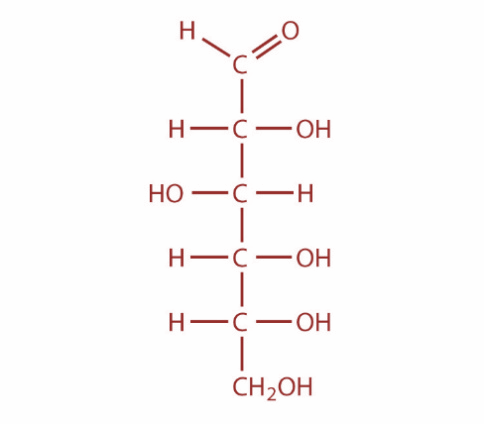
So, both assertion and reason are correct but reason is not the correct explanation for assertion.
Hence, option B is correct.
Note: There is one major difference between starch and cellulose. So, for starch, the glucose repeating units are located in the same direction and each successive glucose unit is rotated 180 degrees in cellulose. Moreover, cellulose applications are found in rayon substitute, cellophane etc.
Complete step by step answer:
Cellulose is a complex carbohydrate with chemical formula ${({C_6}{H_{10}}{O_5})_n}$ . It is odorless and insoluble in water and most organic solvents. On the other hand, starch is weaker than cellulose and can be dissolved in warm water. Further, cellulose is not suitable for human consumption.
Now, osazones are a class of carbohydrates derivatives found in organic chemistry which are formed when reducing sugars are reacted with excess phenylhydrazine at boiling temperatures. This reaction was developed by famous German chemist Emil Fischer. Now, cellulose and starch cannot form osazone. This is because the aldehyde group is not free and it is involved in the formation of glycosidic linkage. Moreover, the monomer of cellulose is D-Glucose and the monomer of Starch is D-glucose. The structure of glucose is as shown:

So, both assertion and reason are correct but reason is not the correct explanation for assertion.
Hence, option B is correct.
Note: There is one major difference between starch and cellulose. So, for starch, the glucose repeating units are located in the same direction and each successive glucose unit is rotated 180 degrees in cellulose. Moreover, cellulose applications are found in rayon substitute, cellophane etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




