
Aspirin is an acetylation product of?
(A) p-dihydroxybenzene
(B) o-hydroxybenzoic acid
(C) o-dihydroxy benzene
(D) m-hydroxybenzoic acid
Answer
586.5k+ views
Hint: Aspirin is also known as Acetylsalicylic acid. We can say that if we do acetylation of Salicylic acid, then we can obtain Aspirin. Acetylation of the reactant brings acetyl group in the product.
Complete step by step answer:
Let us first understand what Aspirin is.
- Aspirin is a NSAID (non-steroidal anti-inflammatory drug), and sometimes it is synthesized from modification of other related compounds called salicylates.
- It is the acetylated form of salicylic acid, with which it was first extracted from a willow bark. Willow bark had long been known to ease pain, reduce fever, and reduce inflammation, and ASA is one of the reasons.
Let us understand the mechanism of aspirin;
- Salicylic acid (O-hydroxybenzoic acid) is a monohydroxybenzoic acid that is a benzoic acid with hydroxyl group at the ortho position. It contains both the organic acid and the phenolic functional groups and is capable of two different esterification reactions, depending on which functional group reacts.
- With an excess of acetic anhydride in the presence of a catalyst (sulphuric acid) to produce the acetylsalicylic acid.
- After the reaction takes place, water is added to destroy the excess acetic anhydride present and cause the product to crystallize.
Synthesis of Aspirin:
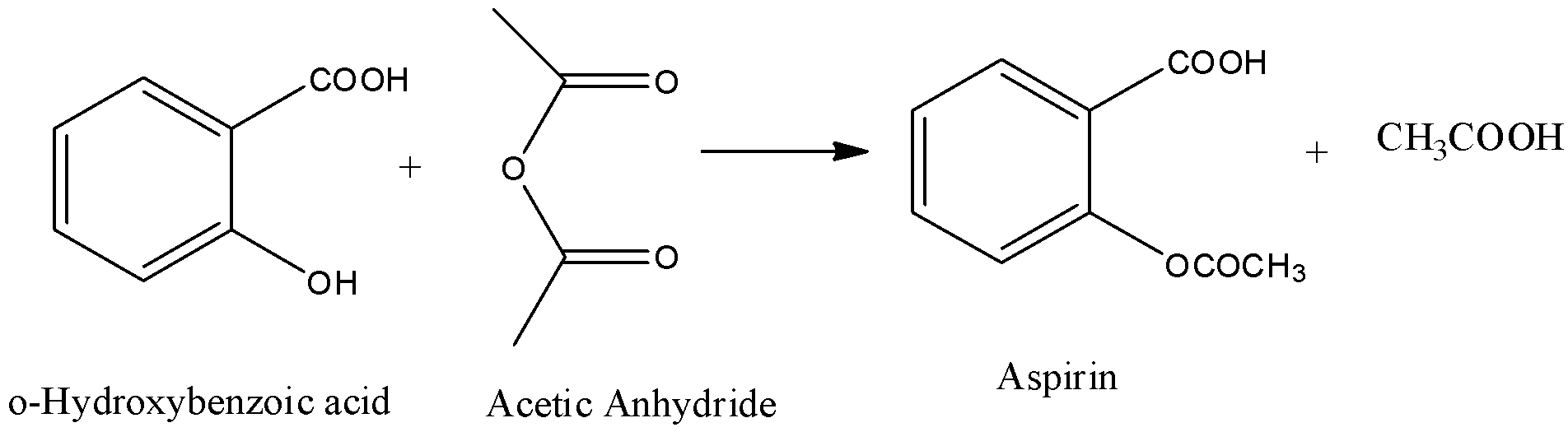
Thus, from the reaction, we can see that we can obtain Aspirin from o-hydroxybenzoic acid by acetylation carried out by acetic anhydride.
- So, the correct answer of this question is (B) o-hydroxybenzoic acid.
Note: Remember that o-hydroxybenzoic acid is also known as Salicylic acid which is its common name. meta and para derivatives of hydroxybenzoic acid will not give Aspirin on acetylation reaction.
Complete step by step answer:
Let us first understand what Aspirin is.
- Aspirin is a NSAID (non-steroidal anti-inflammatory drug), and sometimes it is synthesized from modification of other related compounds called salicylates.
- It is the acetylated form of salicylic acid, with which it was first extracted from a willow bark. Willow bark had long been known to ease pain, reduce fever, and reduce inflammation, and ASA is one of the reasons.
Let us understand the mechanism of aspirin;
- Salicylic acid (O-hydroxybenzoic acid) is a monohydroxybenzoic acid that is a benzoic acid with hydroxyl group at the ortho position. It contains both the organic acid and the phenolic functional groups and is capable of two different esterification reactions, depending on which functional group reacts.
- With an excess of acetic anhydride in the presence of a catalyst (sulphuric acid) to produce the acetylsalicylic acid.
- After the reaction takes place, water is added to destroy the excess acetic anhydride present and cause the product to crystallize.
Synthesis of Aspirin:

Thus, from the reaction, we can see that we can obtain Aspirin from o-hydroxybenzoic acid by acetylation carried out by acetic anhydride.
- So, the correct answer of this question is (B) o-hydroxybenzoic acid.
Note: Remember that o-hydroxybenzoic acid is also known as Salicylic acid which is its common name. meta and para derivatives of hydroxybenzoic acid will not give Aspirin on acetylation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




