
Aspartame is unstable at cooking temperature. Where would you suggest aspartame to be used for sweetening?
(A) Soft drinks
(B) Cold drinks
(C) Both of them
(D) None of these
Answer
588.6k+ views
Hint: Artificial sweeteners are the chemicals that are added to foods and beverages to make them sweet in taste, they are also called sugar substitutes. Artificial sweeteners are used for weight loss, dental problems and in case of hyperglycemia.These artificial sweeteners are generally stable at high temperature and are used while cooking.
Complete step by step answer:
Aspartame is a non-saccharide artificial sweetener. It is around $200$ times sweeter than cane sugar. It was synthesized in $1965$ in the US. It is a methyl ester of the aspartic acid. The structure of aspartame is
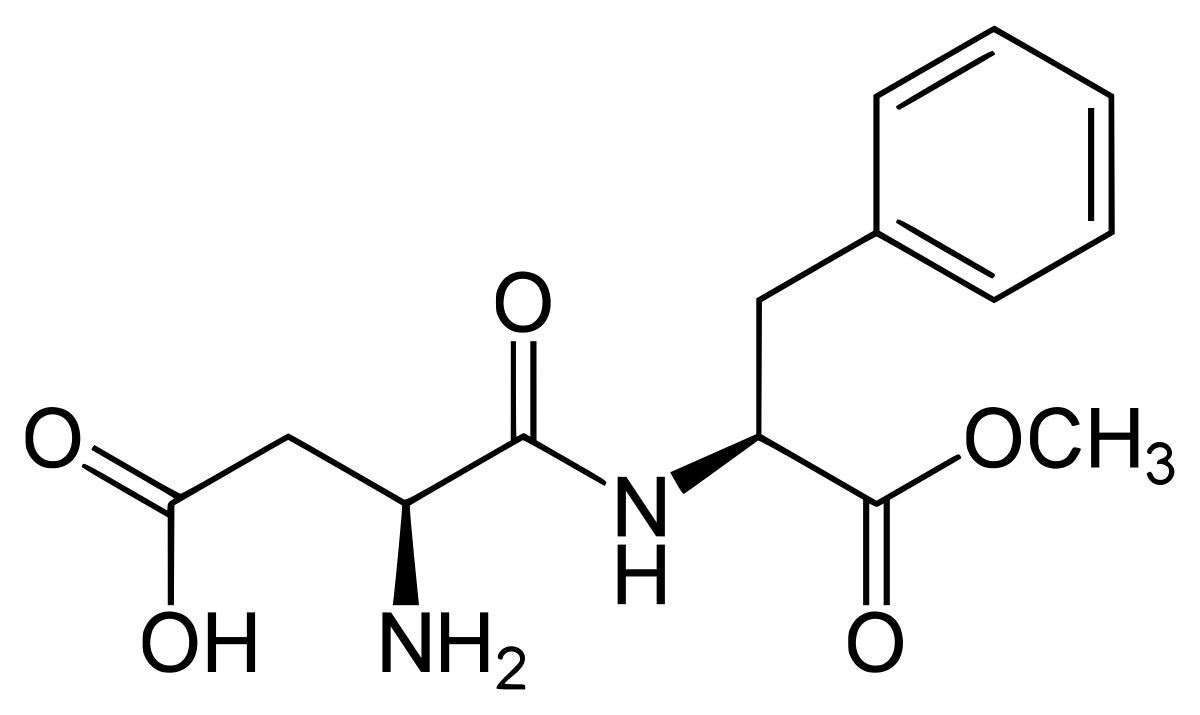
Aspartame is broken down in the small intestines very quickly into residues such as aspartic acid, phenylalanine and methanol, it further breaks down into formaldehyde and formic acid. Aspartame is used as an ingredient in thousands of foods and beverages around the world. It includes diet sodas, soft drinks, instant breakfasts, sugar-free chewing gum, frozen desserts, gelatin made desserts, packed fruit juices, vitamin tablets, milk drinks, medicines, tabletop sweeteners, teas, coffees and yogurt etc. Aspartame is unsuitable for baking and cooking at high temperatures as compared to the other sweeteners as it breaks down on heating and loses the sweetness. The melting point of aspartame is very low, thus it is suitable mainly for foods and beverages at low temperatures. It is ideal for use in cold drinks and soft drinks as these beverages have low temperature.
Hence,the correct option is (C) both of them.
Additional information:
Aspartame is sold in the market under the trade names Equal, NutraSweet,Canderel etc.. Aspartame is one of the most controversial sweeteners in the world. It is said that aspartame can cause health problems from headaches to even cancer. But food safety authorities say it is safe for consumption.
Note:
Aspartame should be used in less quantity as it can be harmful if the intake is in excess as aspartame is high in calories and has no nutritional value. On excess intake it may even lead to some serious diseases.
Complete step by step answer:
Aspartame is a non-saccharide artificial sweetener. It is around $200$ times sweeter than cane sugar. It was synthesized in $1965$ in the US. It is a methyl ester of the aspartic acid. The structure of aspartame is

Aspartame is broken down in the small intestines very quickly into residues such as aspartic acid, phenylalanine and methanol, it further breaks down into formaldehyde and formic acid. Aspartame is used as an ingredient in thousands of foods and beverages around the world. It includes diet sodas, soft drinks, instant breakfasts, sugar-free chewing gum, frozen desserts, gelatin made desserts, packed fruit juices, vitamin tablets, milk drinks, medicines, tabletop sweeteners, teas, coffees and yogurt etc. Aspartame is unsuitable for baking and cooking at high temperatures as compared to the other sweeteners as it breaks down on heating and loses the sweetness. The melting point of aspartame is very low, thus it is suitable mainly for foods and beverages at low temperatures. It is ideal for use in cold drinks and soft drinks as these beverages have low temperature.
Hence,the correct option is (C) both of them.
Additional information:
Aspartame is sold in the market under the trade names Equal, NutraSweet,Canderel etc.. Aspartame is one of the most controversial sweeteners in the world. It is said that aspartame can cause health problems from headaches to even cancer. But food safety authorities say it is safe for consumption.
Note:
Aspartame should be used in less quantity as it can be harmful if the intake is in excess as aspartame is high in calories and has no nutritional value. On excess intake it may even lead to some serious diseases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




