
Arrange the following structure in the increasing order of their stability:
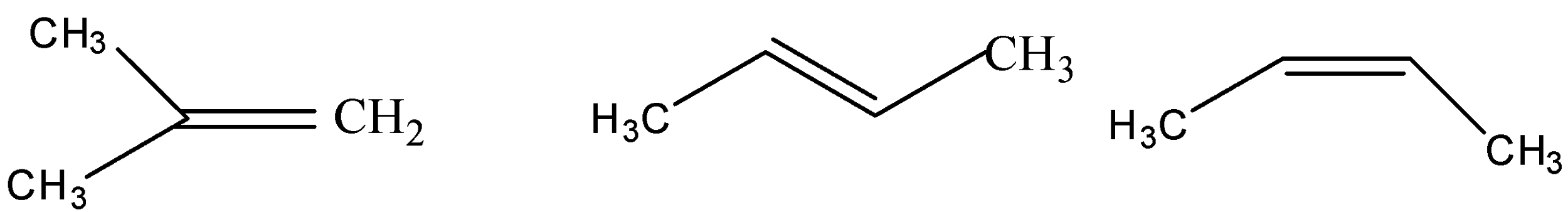
(i) (ii) (iii)

Answer
593.7k+ views
Hint:
When two or more molecules having the same molecular formula differ in their structures or spatial arrangement due to which their physical and chemical properties alter are known as isomers and the phenomenon is known as isomerism. The two isomers differ in their chemical structures or the spatial arrangement of the atoms or groups of atoms present in their molecules. Due to the difference in the chemical structure, we get structural isomerism whereas due to different spatial arrangements we get stereoisomerism.
Complete answer:
Step 1
Structural isomerism is of the following types:
Chain isomerism – When two or more compounds have a similar molecular formula but have a different arrangement of carbon atoms in straight or branched chains are called chain isomers. In the given question structure (I) is the branched isomer whereas structures (II) and (III) are the chain isomers of the compound butene having a molecular formula \[{C_4}{H_8}\].
Position isomerism – In this type of isomerism the position of the double bonds, triple bonds, or the functional groups changes in the different isomers. In the given question structure (I) is but-1-ene whereas structures (II) and (III) are but-2-ene.
Functional isomerism – in this type of isomerism the isomers have the same molecular formula but different functional groups.
Metamerism – in this type of isomerism the isomers have different bulk of alkyl groups attached to both sides of the functional group.
Ring chain isomerism – in this type of isomerism the two isomers are alkene and a cyclic alkane.
Tautomerism – It is the phenomenon in which a single compound exists in two readily interconvertible forms differing in the position of the hydrogen atom.
Step 2
When two compounds having the same molecular form differ in the spatial arrangement of the atoms or groups about double-bonded carbon atoms, the isomers are known as cis-trans isomers. When the similar groups are arranged on the same side of the double bond is called cis isomer and when the similar groups are arranged on the opposite side around the double bond is called the trans isomer. In the given question structure (II) is a trans isomer and structure (III) is the cis isomer.
Step 3
As the structure of the branched isomer is more compact, this results in lowering the energy thereby increasing the stability. So, a branched isomer is more stable than the chain isomers. So, structure (I) is more stable than structures (II) and (III).
Step 4
Cis isomers are less stable than the trans isomers as the bulky groups lie on the same side leading to steric hindrance. So, structure (II) is more stable than the structure (III).
Hence, the increasing order of the stability of the different structures are (III) < (II) < (I).
Note:
The restricted rotation of carbon atoms about a double bond is the main cause of geometrical isomerism.
When two atoms or groups of atoms in an organic compound are at a distance less than or equal to the sum of their van der Waals’ radii, they repel each other due to spatial crowding. This repulsion is known as a steric hindrance.
When two or more molecules having the same molecular formula differ in their structures or spatial arrangement due to which their physical and chemical properties alter are known as isomers and the phenomenon is known as isomerism. The two isomers differ in their chemical structures or the spatial arrangement of the atoms or groups of atoms present in their molecules. Due to the difference in the chemical structure, we get structural isomerism whereas due to different spatial arrangements we get stereoisomerism.
Complete answer:
Step 1
Structural isomerism is of the following types:
Chain isomerism – When two or more compounds have a similar molecular formula but have a different arrangement of carbon atoms in straight or branched chains are called chain isomers. In the given question structure (I) is the branched isomer whereas structures (II) and (III) are the chain isomers of the compound butene having a molecular formula \[{C_4}{H_8}\].
Position isomerism – In this type of isomerism the position of the double bonds, triple bonds, or the functional groups changes in the different isomers. In the given question structure (I) is but-1-ene whereas structures (II) and (III) are but-2-ene.
Functional isomerism – in this type of isomerism the isomers have the same molecular formula but different functional groups.
Metamerism – in this type of isomerism the isomers have different bulk of alkyl groups attached to both sides of the functional group.
Ring chain isomerism – in this type of isomerism the two isomers are alkene and a cyclic alkane.
Tautomerism – It is the phenomenon in which a single compound exists in two readily interconvertible forms differing in the position of the hydrogen atom.
Step 2
When two compounds having the same molecular form differ in the spatial arrangement of the atoms or groups about double-bonded carbon atoms, the isomers are known as cis-trans isomers. When the similar groups are arranged on the same side of the double bond is called cis isomer and when the similar groups are arranged on the opposite side around the double bond is called the trans isomer. In the given question structure (II) is a trans isomer and structure (III) is the cis isomer.
Step 3
As the structure of the branched isomer is more compact, this results in lowering the energy thereby increasing the stability. So, a branched isomer is more stable than the chain isomers. So, structure (I) is more stable than structures (II) and (III).
Step 4
Cis isomers are less stable than the trans isomers as the bulky groups lie on the same side leading to steric hindrance. So, structure (II) is more stable than the structure (III).
Hence, the increasing order of the stability of the different structures are (III) < (II) < (I).
Note:
The restricted rotation of carbon atoms about a double bond is the main cause of geometrical isomerism.
When two atoms or groups of atoms in an organic compound are at a distance less than or equal to the sum of their van der Waals’ radii, they repel each other due to spatial crowding. This repulsion is known as a steric hindrance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




