
Arrange the following in the increasing order of their acidic character: Benzoic acid, phenol, cresol
Answer
577.8k+ views
Hint: This question can be answered on the basis of inductive effect. The induction of polarity between two atoms where one is more electronegative and the other is less electronegative is known as inductive effect.
Complete answer:
A covalent is known to be formed by sharing if electrons between two atoms. If the electronegativity of two atoms that are sharing a bond between them are different. The electron pair forming the sigma bond is not equally shared between the two atoms. Here, the electron pair gets displaced towards the more electronegative of the two. Due to this displacement a partial negative charge on the more electronegative atom and an equivalent partial positive charge on the other atom. This induction of polarity in a covalent bond and this is called the inductive effect.
It is represented by the symbol (
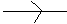 ) that is the arrowhead pointing towards the more electronegative atom or group.
) that is the arrowhead pointing towards the more electronegative atom or group.
Let’s see what is –I effect and +I effect,
When the substituent linked to the carbon atom is electron attracting (X) then, it is said to exert a negative inductive effect.
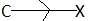
When the substituent linked to the carbon atom is electron releasing then, it is said to exert a positive inductive effect.
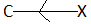
Some of the common groups or substituent responsible for –I effect are given below in decreasing order. This series is also known as –I series.
\[ - N{O_2} > - CN > - COH > - COOH > - F > - Cl > - Br > - I > - OR > - OH > - {C_6}{H_5} > - H\]
With increase in –I effect, the acidic nature also increases in the same trend. Benzoic acid chemical formula is ${C_6}{H_5}COOH$. It has –COOH group which exerts more –the effect is that it attracts the aliphatic or aromatic group to which it is attached towards itself. With reference to the series illustrated, we can come to a conclusion that it is more acidic than phenol as it has an OH group. The chemical formula is ${C_6}{H_5}OH$. The chemical formula of Cresol is $C{H_2}{C_5}{H_4}OH$. It has two substitutes, where one is –OH and the other one is$ - C{H_2}$. The presence of $ - C{H_2}$ group is an electron releasing group so it decreases the acidic nature of cresol
Thus, the correct order is :Cresol < phenol < benzoic acid.
Note:
With increase in +I affect the basicity also increase. $ - C{H_2}$ exerts +I effect and $ - C{H_2}$ comes first in +I series. So, there is a decrease in the acidic nature of cresol. The PKa of cresol is around 10 which is actually basic.
Complete answer:
A covalent is known to be formed by sharing if electrons between two atoms. If the electronegativity of two atoms that are sharing a bond between them are different. The electron pair forming the sigma bond is not equally shared between the two atoms. Here, the electron pair gets displaced towards the more electronegative of the two. Due to this displacement a partial negative charge on the more electronegative atom and an equivalent partial positive charge on the other atom. This induction of polarity in a covalent bond and this is called the inductive effect.
It is represented by the symbol (

Let’s see what is –I effect and +I effect,
When the substituent linked to the carbon atom is electron attracting (X) then, it is said to exert a negative inductive effect.
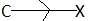
When the substituent linked to the carbon atom is electron releasing then, it is said to exert a positive inductive effect.

Some of the common groups or substituent responsible for –I effect are given below in decreasing order. This series is also known as –I series.
\[ - N{O_2} > - CN > - COH > - COOH > - F > - Cl > - Br > - I > - OR > - OH > - {C_6}{H_5} > - H\]
With increase in –I effect, the acidic nature also increases in the same trend. Benzoic acid chemical formula is ${C_6}{H_5}COOH$. It has –COOH group which exerts more –the effect is that it attracts the aliphatic or aromatic group to which it is attached towards itself. With reference to the series illustrated, we can come to a conclusion that it is more acidic than phenol as it has an OH group. The chemical formula is ${C_6}{H_5}OH$. The chemical formula of Cresol is $C{H_2}{C_5}{H_4}OH$. It has two substitutes, where one is –OH and the other one is$ - C{H_2}$. The presence of $ - C{H_2}$ group is an electron releasing group so it decreases the acidic nature of cresol
Thus, the correct order is :Cresol < phenol < benzoic acid.
Note:
With increase in +I affect the basicity also increase. $ - C{H_2}$ exerts +I effect and $ - C{H_2}$ comes first in +I series. So, there is a decrease in the acidic nature of cresol. The PKa of cresol is around 10 which is actually basic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




