
Arrange the following amines in decreasing order of basicity:
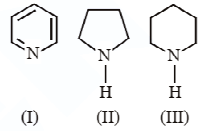
a.) \[II\]>\[I\]>\[III\]
b.) \[III\]>\[II\]>\[I\]
c.) \[I\]>\[II\]>\[III\]
d.) \[III\]>\[I\]>\[II\]

Answer
598.2k+ views
Hint: This question brings into light the basicity of amines. In this question we will cover up content like the basic character of amines, what influences it, and structure – basicity relationship and all the related information. And using this information, we will be approaching our answer. Below here we have discussed everything properly.
Complete step by step solution: Basicity of Amines: Amines have an unshared pair of electrons over the nitrogen atom and hence, they behave as Lewis Base. The reaction of amines with mineral acid will form ammonium salts which shows that these are basic in nature.
\[p{K_b} = - \log {K_b}\]
As there will be a rise in the value of \[{K_b}\] or if there is a fall in \[p{K_b}\] value. The power of the base will rise.
Aromatic amines are less basic than that of ammonia as there is a presence of electron withdrawing groups. Hence, we can say electron withdrawing groups have a negative impact on the basicity of aryl amines.
Structure-basicity relationship: basic character of amine depends on the fact that how speedily and without hindrance formation of cation by accepting a proton from the acid takes place.
The rise in stability has a direct effect on the basicity of amines.
Aryl amines V/S Ammonia: \[p{K_b}\] value of aniline is quite high, as the ammonia group is directly attached to the benzene ring. So as a result of this unshared pair of electrons start to be in conjugation with the benzene ring.
Thus, at last making it less free for protonation. Aniline has five resonating structures which gives us information that it is more stable than ammonia. So aniline is more basic.
Basic strength rises because electron donating capacity of nitrogen rises.
In compound III, the nitrogen has two lone pairs of electrons for donation whereas, in compound II, the lone pair of electrons are delocalized in the aromatic ring.
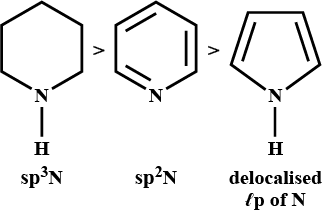
So, by this we can conclude that option (B) is the correct answer.
Hence, the correct option is B.
Note: In this question we learned the basicity of amines and factors that govern it. If we talk of alkyl amines there are very many changes in their order of reactivity due to phases but in aryl amines this is not the condition.
Complete step by step solution: Basicity of Amines: Amines have an unshared pair of electrons over the nitrogen atom and hence, they behave as Lewis Base. The reaction of amines with mineral acid will form ammonium salts which shows that these are basic in nature.
\[p{K_b} = - \log {K_b}\]
As there will be a rise in the value of \[{K_b}\] or if there is a fall in \[p{K_b}\] value. The power of the base will rise.
Aromatic amines are less basic than that of ammonia as there is a presence of electron withdrawing groups. Hence, we can say electron withdrawing groups have a negative impact on the basicity of aryl amines.
Structure-basicity relationship: basic character of amine depends on the fact that how speedily and without hindrance formation of cation by accepting a proton from the acid takes place.
The rise in stability has a direct effect on the basicity of amines.
Aryl amines V/S Ammonia: \[p{K_b}\] value of aniline is quite high, as the ammonia group is directly attached to the benzene ring. So as a result of this unshared pair of electrons start to be in conjugation with the benzene ring.
Thus, at last making it less free for protonation. Aniline has five resonating structures which gives us information that it is more stable than ammonia. So aniline is more basic.
Basic strength rises because electron donating capacity of nitrogen rises.
In compound III, the nitrogen has two lone pairs of electrons for donation whereas, in compound II, the lone pair of electrons are delocalized in the aromatic ring.

So, by this we can conclude that option (B) is the correct answer.
Hence, the correct option is B.
Note: In this question we learned the basicity of amines and factors that govern it. If we talk of alkyl amines there are very many changes in their order of reactivity due to phases but in aryl amines this is not the condition.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




