
How are $Xe{{F}_{2}}$ and $Xe{{F}_{4}}$ prepared? Give their structures.
Answer
590.4k+ views
Hint: Think about the electronic configuration of the $Xe$ atoms present in both the compounds and try to figure out its hybridization to form the given compounds. Take into consideration any charge that may be present on the molecule.
Complete step by step answer:
We know that $Xe$ is an atom that is present in the group number 18 of the periodic table that usually contains noble gases that do not react due to completely filled orbitals and a stable configuration. So, we can deduce that it only forms bonds by donation of a lone pair of electrons or by promoting electrons to the higher empty d-orbital and then hybridizing the required amount of orbitals. Instead of the former, the latter phenomenon of hybridization takes place for increased stability of the molecule. The electronic configuration of $Xe$ is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Remember that the atom has an empty $5d$ orbital.
The reactions that takes place for the formation of the molecules $Xe{{F}_{2}}$ and $Xe{{F}_{4}}$ are as follows:
- For $Xe{{F}_{2}}$
\[X{{e}_{(g)}}+{{F}_{2}}_{(g)}\xrightarrow{673K,1bar}Xe{{F}_{2}}_{(s)}\]
Here, $Xe$ has to be present in excess so that it actually reacts with fluorine and does not just retain its noble gas configuration.
- For $Xe{{F}_{4}}$
\[X{{e}_{(g)}}+2{{F}_{2}}_{(g)}\xrightarrow{873K,7bar}Xe{{F}_{4}}_{(s)}\]
Here, $Xe$ and $F$ has to be present in the proportion 1:5 since we do not want to stop at the formation of $Xe{{F}_{2}}$ but want xenon too continue to bond with fluorine to form $Xe{{F}_{4}}$.
Now, moving on to the configuration; the original configuration of $Xe$ since it does not have any charge is:
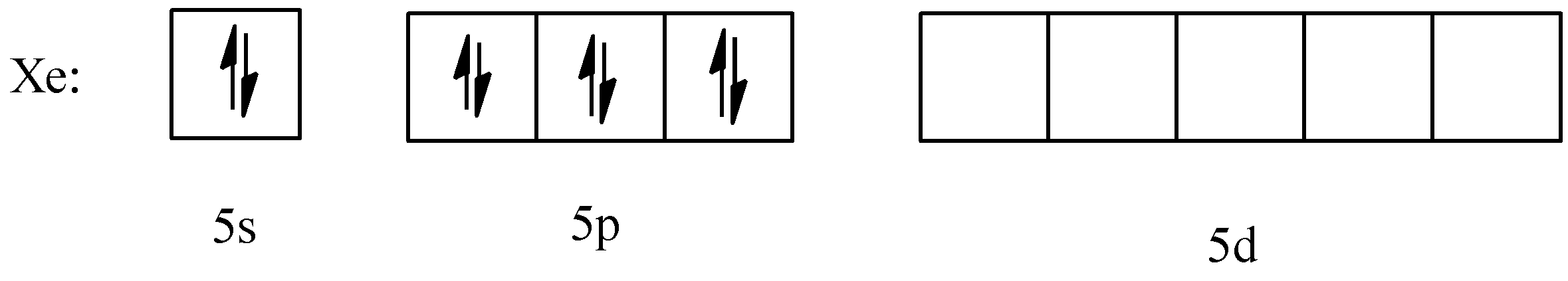
Now, from this ground state, the atom hybridizes to form unpaired electrons and bond with fluorine.
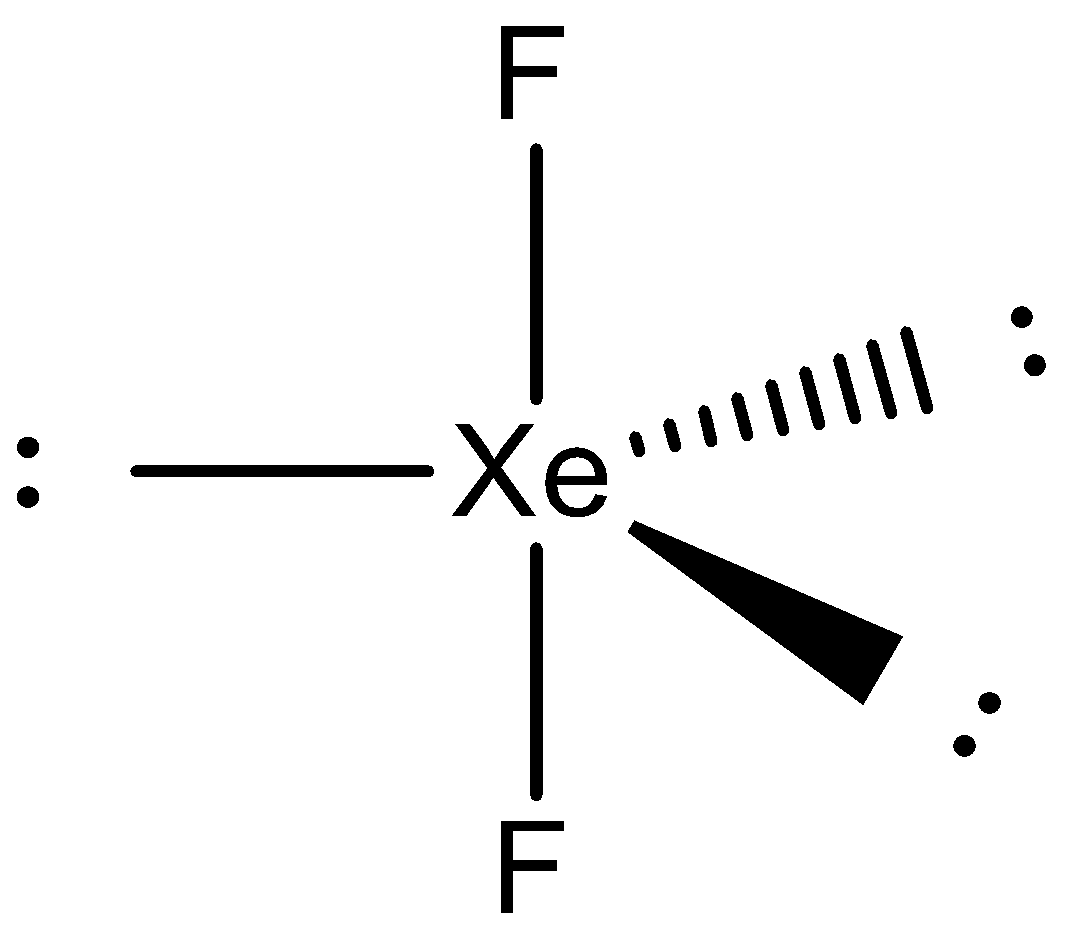
- For $Xe{{F}_{2}}$
Here, xenon moves one electron from the p-orbital to the d-orbital, to form 2 unpaired electrons. The orbitals then hybridize to form 5 $s{{p}^{3}}d$ orbitals which arrange themselves in the geometry of trigonal bipyramidal. The atom has 3 lone pairs of electrons that take the equatorial positions and 2 bond pairs that take the axial positions. So, the visible geometry of this molecule will be linear. The structure is as follows:
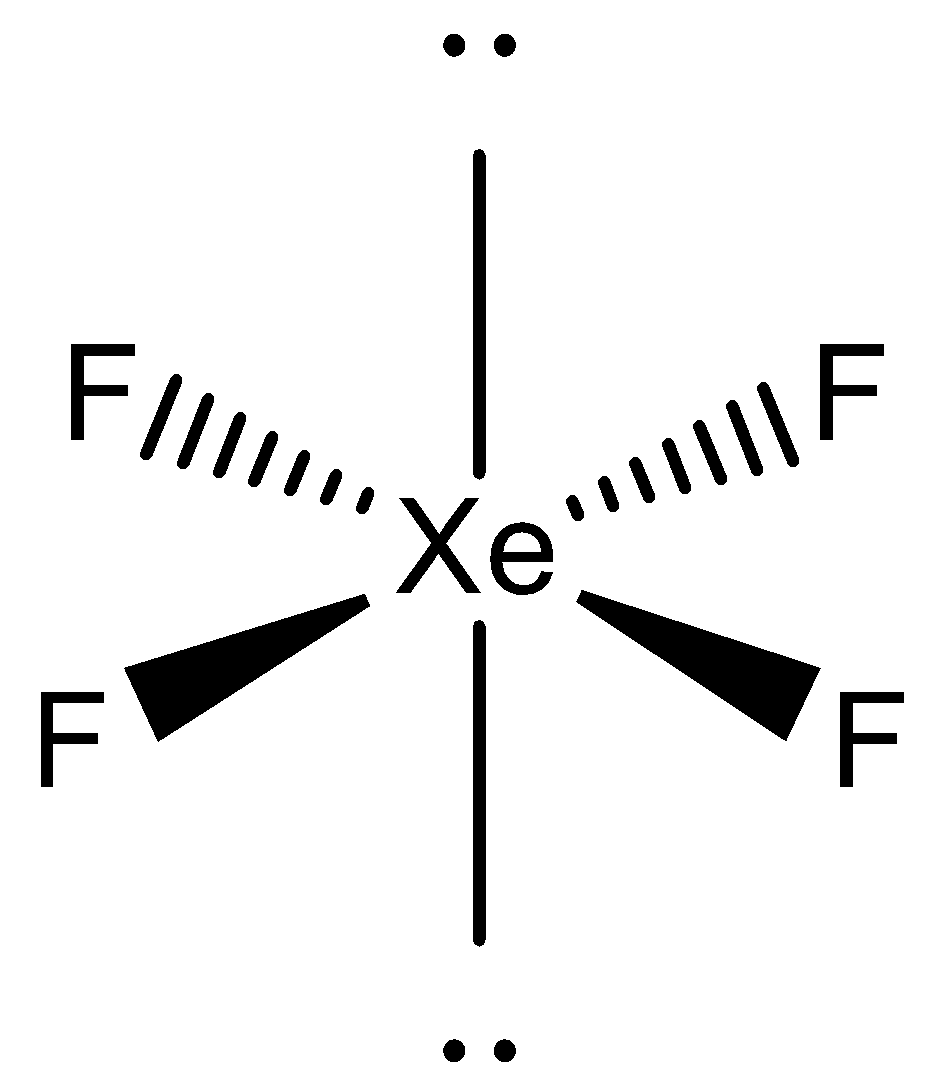
- For $Xe{{F}_{4}}$
Here, xenon moves two electrons from the p-orbital to the d-orbital, to form 4 unpaired electrons. The orbitals then hybridize to form 6 $s{{p}^{3}}{{d}^{2}}$ orbitals which arrange themselves in the octahedral geometry. The atom has 2 lone pairs of electrons that take the axial positions and 4 bond pairs that take the equatorial positions. So, the visible geometry of this molecule will be square planar. The structure is as follows:
Note: The formation of $Xe{{F}_{6}}$ can also take place by a similar reaction but to balance it, 3 moles of fluorine gas will be required. The reagents will have to be mixed in the proportion of 1:20 for $Xe:F$ since we want it to react to form more bonds and not get stuck at the molecules $Xe{{F}_{4}}$ or $Xe{{F}_{2}}$. The reaction conditions required are 573 K and 60-70 bars.
Complete step by step answer:
We know that $Xe$ is an atom that is present in the group number 18 of the periodic table that usually contains noble gases that do not react due to completely filled orbitals and a stable configuration. So, we can deduce that it only forms bonds by donation of a lone pair of electrons or by promoting electrons to the higher empty d-orbital and then hybridizing the required amount of orbitals. Instead of the former, the latter phenomenon of hybridization takes place for increased stability of the molecule. The electronic configuration of $Xe$ is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Remember that the atom has an empty $5d$ orbital.
The reactions that takes place for the formation of the molecules $Xe{{F}_{2}}$ and $Xe{{F}_{4}}$ are as follows:
- For $Xe{{F}_{2}}$
\[X{{e}_{(g)}}+{{F}_{2}}_{(g)}\xrightarrow{673K,1bar}Xe{{F}_{2}}_{(s)}\]
Here, $Xe$ has to be present in excess so that it actually reacts with fluorine and does not just retain its noble gas configuration.
- For $Xe{{F}_{4}}$
\[X{{e}_{(g)}}+2{{F}_{2}}_{(g)}\xrightarrow{873K,7bar}Xe{{F}_{4}}_{(s)}\]
Here, $Xe$ and $F$ has to be present in the proportion 1:5 since we do not want to stop at the formation of $Xe{{F}_{2}}$ but want xenon too continue to bond with fluorine to form $Xe{{F}_{4}}$.
Now, moving on to the configuration; the original configuration of $Xe$ since it does not have any charge is:

Now, from this ground state, the atom hybridizes to form unpaired electrons and bond with fluorine.
- For $Xe{{F}_{2}}$
Here, xenon moves one electron from the p-orbital to the d-orbital, to form 2 unpaired electrons. The orbitals then hybridize to form 5 $s{{p}^{3}}d$ orbitals which arrange themselves in the geometry of trigonal bipyramidal. The atom has 3 lone pairs of electrons that take the equatorial positions and 2 bond pairs that take the axial positions. So, the visible geometry of this molecule will be linear. The structure is as follows:

- For $Xe{{F}_{4}}$
Here, xenon moves two electrons from the p-orbital to the d-orbital, to form 4 unpaired electrons. The orbitals then hybridize to form 6 $s{{p}^{3}}{{d}^{2}}$ orbitals which arrange themselves in the octahedral geometry. The atom has 2 lone pairs of electrons that take the axial positions and 4 bond pairs that take the equatorial positions. So, the visible geometry of this molecule will be square planar. The structure is as follows:

Note: The formation of $Xe{{F}_{6}}$ can also take place by a similar reaction but to balance it, 3 moles of fluorine gas will be required. The reagents will have to be mixed in the proportion of 1:20 for $Xe:F$ since we want it to react to form more bonds and not get stuck at the molecules $Xe{{F}_{4}}$ or $Xe{{F}_{2}}$. The reaction conditions required are 573 K and 60-70 bars.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




