
What are the two functional groups in glucose?
Answer
501.3k+ views
Hint: Glucose is an important biomolecule that is considered to be the simplest form of carbohydrates present in the body. Glucose is a monosaccharide that forms many complex polysaccharides sugars and provides energy to the body.
Complete answer:
Glucose is an organic molecule consisting of six carbon atoms in the parent chain connected to each other through single bonds and six oxygen atoms. The molecular formula of glucose is: \[{C_6}{H_{12}}{O_6}\] . The structure of glucose can be shown as follows:
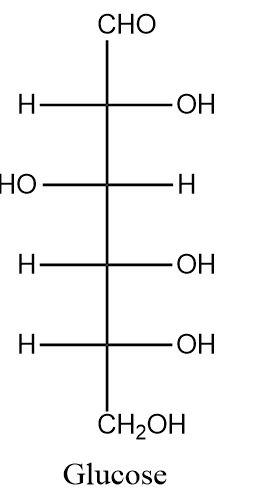
The given structure of glucose is a straight chain structure. Fischer and Haworth gave other forms of glucose in which it forms a six membered ring with one of the members being an oxygen atom. Such structures do not give information about the functional groups as the aldehyde group of glucose is not present in its free form in these six-membered rings.
Glucose is a pentahydroxy molecule i.e. five out of six carbon atoms in the structure of glucose are connected to the hydroxyl functional group and the topmost carbon atom is present in the most oxidized form which is the aldehyde form.
Thus, the two functional groups present in glucose molecules are the aldehyde group \[( - CHO)\] and the hydroxyl group \[( - OH)\] and the correct option is (ii).
Note:
The structure of fructose and glucose is nearly the same as both of them are monosaccharide molecules containing six carbon atoms each. The only factor that helps us distinguish between their structures is that glucose contains an aldehyde functional group while fructose contains a ketone group.
Complete answer:
Glucose is an organic molecule consisting of six carbon atoms in the parent chain connected to each other through single bonds and six oxygen atoms. The molecular formula of glucose is: \[{C_6}{H_{12}}{O_6}\] . The structure of glucose can be shown as follows:

The given structure of glucose is a straight chain structure. Fischer and Haworth gave other forms of glucose in which it forms a six membered ring with one of the members being an oxygen atom. Such structures do not give information about the functional groups as the aldehyde group of glucose is not present in its free form in these six-membered rings.
Glucose is a pentahydroxy molecule i.e. five out of six carbon atoms in the structure of glucose are connected to the hydroxyl functional group and the topmost carbon atom is present in the most oxidized form which is the aldehyde form.
Thus, the two functional groups present in glucose molecules are the aldehyde group \[( - CHO)\] and the hydroxyl group \[( - OH)\] and the correct option is (ii).
Note:
The structure of fructose and glucose is nearly the same as both of them are monosaccharide molecules containing six carbon atoms each. The only factor that helps us distinguish between their structures is that glucose contains an aldehyde functional group while fructose contains a ketone group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




