
What are the total number of stereoisomers of $ 2,3 - $ dichlorobutane?
Answer
495.3k+ views
Hint: Isomerism is the property of chemical compounds in which compounds can exist in two different forms but having the same molecular formula. Stereochemistry is a type of isomer in which the position of substituents changes in three-dimensional orientation.
Complete answer:
Stereoisomerism of particular compounds have the same chemical formula, same number of atoms but only spatial arrangements differ. Therefore, stereoisomerism is also known as spatial isomerism.
$ 2,3 - $ dichlorobutane molecule do not give any geometric isomers because geometric isomerism is shown by the compounds having double bonds.
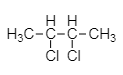
As we see, the $ 2,3 - $ dichlorobutane molecule is symmetrical in nature due to the presence of the same substituent in the end.
$ 2,3 - $ dichlorobutane molecule has a two chiral center hence capable of rotating plane polarized light into right and left direction.
The mathematical formula which is used to calculate the total number of stereoisomers for an even number of chiral centers is;
$ S = {2^{n - 1}} + {2^{n/2 - 1}} $
Where $ S = $ total number of stereoisomers
$ n = $ Total number of chiral centers present in a molecule
As we noticed from the structure there are two chiral centers so the value of $ \left( n \right) $ is $ 2 $ .
Now put the value of $ \left( n \right) $ in the above formula-
$ S = {2^{2 - 1}} + {2^{\dfrac{2}{2} - 1}} $
After solving this equation, we get
$ S = {2^1} + {2^0} $
$ S = 2 + 1 $
Hence, the value of $ S $ after calculation is $ 3 $ .
Hence according to the formula there are a total of three stereoisomers possible for $ 2,3 - $ dichlorobutane.
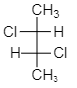
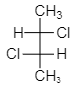
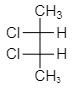
As we see from the above structure, figure $ \left( {III} \right) $ shows the presence of symmetry of a molecule and hence it is mesomeres. As we know mesomeres have net zero optical rotation therefore they are considered as an optically inactive isomer.
Hence the total number of stereoisomers of $ 2,3 - $ dichlorobutane is $ 3 $ .
Note:
Enantiomers or optical isomers are two forms of stereoisomers which are mirror images of each other and are non-superimposable in nature. Diastereomers are two forms of stereoisomers which are non-mirror images of each other and are non-superimposable in nature.
Complete answer:
Stereoisomerism of particular compounds have the same chemical formula, same number of atoms but only spatial arrangements differ. Therefore, stereoisomerism is also known as spatial isomerism.
$ 2,3 - $ dichlorobutane molecule do not give any geometric isomers because geometric isomerism is shown by the compounds having double bonds.
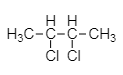
As we see, the $ 2,3 - $ dichlorobutane molecule is symmetrical in nature due to the presence of the same substituent in the end.
$ 2,3 - $ dichlorobutane molecule has a two chiral center hence capable of rotating plane polarized light into right and left direction.
The mathematical formula which is used to calculate the total number of stereoisomers for an even number of chiral centers is;
$ S = {2^{n - 1}} + {2^{n/2 - 1}} $
Where $ S = $ total number of stereoisomers
$ n = $ Total number of chiral centers present in a molecule
As we noticed from the structure there are two chiral centers so the value of $ \left( n \right) $ is $ 2 $ .
Now put the value of $ \left( n \right) $ in the above formula-
$ S = {2^{2 - 1}} + {2^{\dfrac{2}{2} - 1}} $
After solving this equation, we get
$ S = {2^1} + {2^0} $
$ S = 2 + 1 $
Hence, the value of $ S $ after calculation is $ 3 $ .
Hence according to the formula there are a total of three stereoisomers possible for $ 2,3 - $ dichlorobutane.



As we see from the above structure, figure $ \left( {III} \right) $ shows the presence of symmetry of a molecule and hence it is mesomeres. As we know mesomeres have net zero optical rotation therefore they are considered as an optically inactive isomer.
Hence the total number of stereoisomers of $ 2,3 - $ dichlorobutane is $ 3 $ .
Note:
Enantiomers or optical isomers are two forms of stereoisomers which are mirror images of each other and are non-superimposable in nature. Diastereomers are two forms of stereoisomers which are non-mirror images of each other and are non-superimposable in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




