
What are the names of the elements in methane?
A. Carbon and chlorine
B. Carbon and hydrogen
C. Hydrogen and chlorine
D. Oxygen and hydrogen
Answer
493.5k+ views
Hint: In organic chemistry, an alkane which is also known as paraffin is an acyclic saturated hydrocarbon i.e., it consists of hydrogen and carbon atoms covalently bonded to each other via single bonds. The general chemical formula of alkanes is ${C_n}{H_{2n + 2}}$.
Complete answer:
Methane is an organic compound with chemical formula $C{H_4}$. It is the simplest alkane and main constituent of natural gas. It is a tetrahedral molecule with four equivalent $C - H$ bonds. At room temperature and standard pressure, methane is a colourless and odourless gas. Structurally, it is represented as follows:
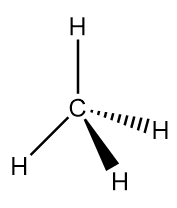
Thus, we can conclude the names of elements of methane are carbon and hydrogen.
Hence, option (B) is the correct answer.
Additional information-
The electronic configuration of a carbon atom is $1{s^2}2{s^2}2{p^2}$ that means only 2 electrons are unpaired in the ground state of the carbon atom. At an excited state, the mixing of orbitals takes place to generate hybrid orbitals with similar energy and electrons are rearranged in order to participate in bonding. This phenomenon is known as hybridization. In methane, the electrons are reorganized into one s and three p orbitals and thus, the carbon atom in methane is $s{p^3}$ hybridized.
Note:
Remember that all the hydrogen atoms are bonded to the carbon atom via covalent bond i.e., the rotation of hydrogen atoms is not restricted. The hydrogen atoms in the methane molecule are allowed to rotate freely and thus, it is also referred to as a plastic crystal.
Complete answer:
Methane is an organic compound with chemical formula $C{H_4}$. It is the simplest alkane and main constituent of natural gas. It is a tetrahedral molecule with four equivalent $C - H$ bonds. At room temperature and standard pressure, methane is a colourless and odourless gas. Structurally, it is represented as follows:

Thus, we can conclude the names of elements of methane are carbon and hydrogen.
Hence, option (B) is the correct answer.
Additional information-
The electronic configuration of a carbon atom is $1{s^2}2{s^2}2{p^2}$ that means only 2 electrons are unpaired in the ground state of the carbon atom. At an excited state, the mixing of orbitals takes place to generate hybrid orbitals with similar energy and electrons are rearranged in order to participate in bonding. This phenomenon is known as hybridization. In methane, the electrons are reorganized into one s and three p orbitals and thus, the carbon atom in methane is $s{p^3}$ hybridized.
Note:
Remember that all the hydrogen atoms are bonded to the carbon atom via covalent bond i.e., the rotation of hydrogen atoms is not restricted. The hydrogen atoms in the methane molecule are allowed to rotate freely and thus, it is also referred to as a plastic crystal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




