
What are the monomers of Dacron?
Answer
510.9k+ views
Hint :The monomers involved in the synthesis of Dacron are ethylene glycol and terephthalic acid. The repeating unit of polymer is called monomer. Condensation polymerization is the process whereby monomers attach to each other via elimination of small molecules such as \[H_2O\], \[HCl\].
Complete Step By Step Answer:
The structure of first monomer i.e. ethylene glycol is given as follows:
\[HOCH_2CH_2OH\]
The structure of second monomer i.e. terephthalic acid is given as follows:
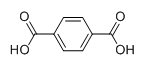
These two monomers joined each other in an alternate manner to synthesize Dacron. The polymerization process for the synthesis of Dacron is illustrate by the following reaction:
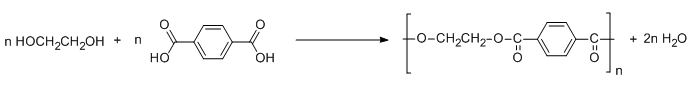
The above reaction takes place at high temperature and in the presence of catalysts such as zinc acetate and antimony trioxide. Herein, esterification takes place with elimination of water. Hence, condensation polymerization occurs between the monomers to enable the synthesis of polyester Dacron.
Therefore, ethylene glycol and terephthalic acid are the monomers of Dacron.
Additional Information:
Dacron is called polyethylene terephthalate. Dacron has high durability, very strong, high resistance to heat and light, and easy to wash. Condensation polymerization is a type of step-growth polymerization. Both monomers i.e. ethylene glycol and terephthalic acid are bifunctional monomers and hence would most likely undergo condensation polymerization. Besides polyester synthesis, condensation polymerization is also involved in the synthesis of polyamides such as Nylon-6, 10.
Note :
It is important to note that ethylene glycol and terephthalic acid are the monomers of Dacron. Condensation polymerization occurs between these monomers to enable the synthesis of polyester Dacron. In this condensation polymerization process, water \[(H2O)\] is eliminated as a by-product.
Complete Step By Step Answer:
The structure of first monomer i.e. ethylene glycol is given as follows:
\[HOCH_2CH_2OH\]
The structure of second monomer i.e. terephthalic acid is given as follows:

These two monomers joined each other in an alternate manner to synthesize Dacron. The polymerization process for the synthesis of Dacron is illustrate by the following reaction:

The above reaction takes place at high temperature and in the presence of catalysts such as zinc acetate and antimony trioxide. Herein, esterification takes place with elimination of water. Hence, condensation polymerization occurs between the monomers to enable the synthesis of polyester Dacron.
Therefore, ethylene glycol and terephthalic acid are the monomers of Dacron.
Additional Information:
Dacron is called polyethylene terephthalate. Dacron has high durability, very strong, high resistance to heat and light, and easy to wash. Condensation polymerization is a type of step-growth polymerization. Both monomers i.e. ethylene glycol and terephthalic acid are bifunctional monomers and hence would most likely undergo condensation polymerization. Besides polyester synthesis, condensation polymerization is also involved in the synthesis of polyamides such as Nylon-6, 10.
Note :
It is important to note that ethylene glycol and terephthalic acid are the monomers of Dacron. Condensation polymerization occurs between these monomers to enable the synthesis of polyester Dacron. In this condensation polymerization process, water \[(H2O)\] is eliminated as a by-product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




