
What are the functional groups in vitamin C?
Answer
524.4k+ views
Hint: Vitamin C , also known as ascorbic acid , is a very essential vitamin which is to be required by our body daily in the diet. If you know about the structure of the vitamin C , then through it you can easily come to know about the functional groups present in it.
Complete answer:
First of all, let's discuss Vitamins. Vitamins are the organic compounds which cannot be produced by the body and must be supplied in small amounts in diet to perform specific biological functions for the normal health, growth and maintenance of the body.
Now coming to vitamin C. The chemical name of the vitamin C is ascorbic acid.
The sources of the vitamin C are amla, tomatoes, mangoes, oranges, pears, pineapple, cabbage , apples, lemon, green chilies etc. amla is a very good source of vitamin C.
The functions of vitamin C are as follows:-
1) It is necessary for keeping teeth, gums and joints healthy.
2) It plays an important role in normal metabolism of the amino acids.
3) It helps in healing of cuts and wounds and gives resistance to our body against diseases and infections.
Now considering the statement as;
The structure of vitamin C is as;-
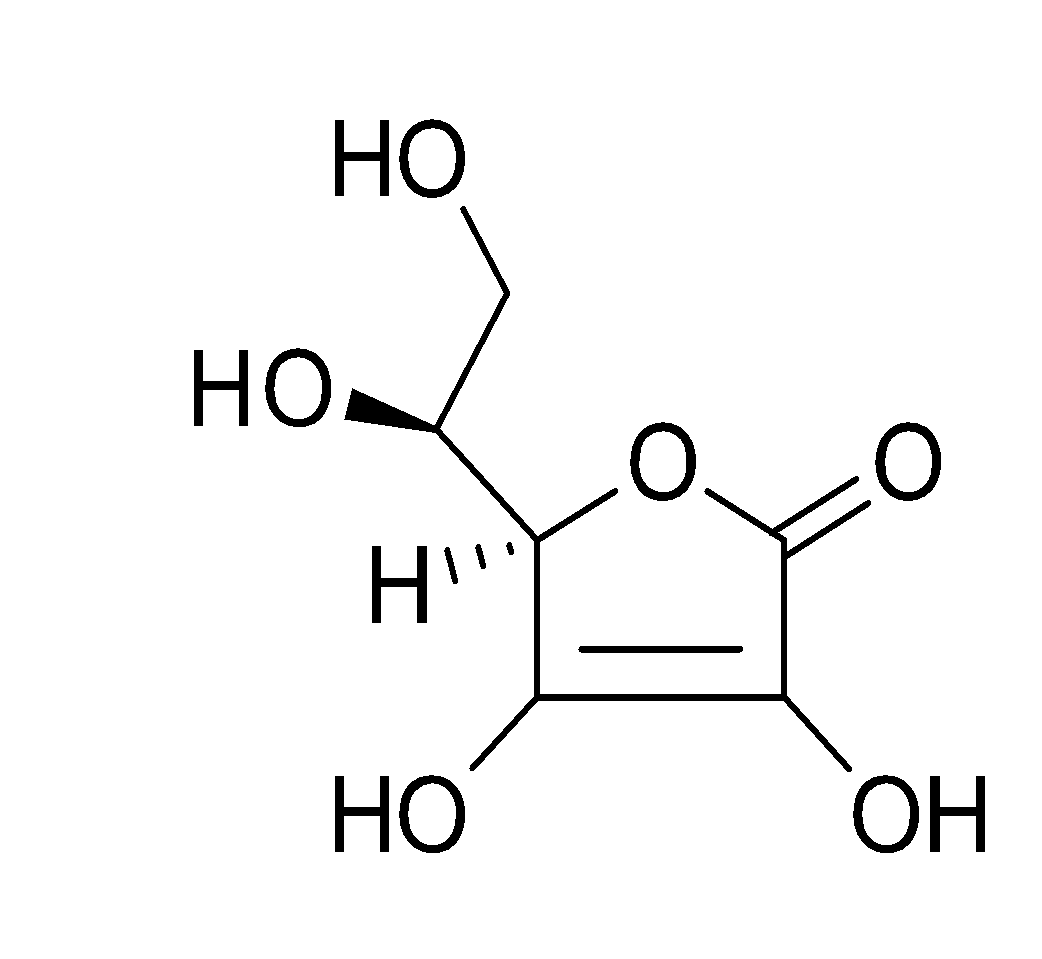
So, thus from the structure of Vitamin C we can see that the functional groups present in it are:- -OH group (i.e. hydroxyl group), -C=O (carbonyl group) and -O- group (i.e. ether group).
Hence, the functional group in vitamin C are hydroxyl, carbonyl and ether respectively.
Note:
Vitamin C is soluble in water and is therefore known as the water-soluble vitamins. Water soluble vitamins are those vitamins which must be supplied in the diet regularly because they are readily excreted in urine and cannot be stored in our body.
Complete answer:
First of all, let's discuss Vitamins. Vitamins are the organic compounds which cannot be produced by the body and must be supplied in small amounts in diet to perform specific biological functions for the normal health, growth and maintenance of the body.
Now coming to vitamin C. The chemical name of the vitamin C is ascorbic acid.
The sources of the vitamin C are amla, tomatoes, mangoes, oranges, pears, pineapple, cabbage , apples, lemon, green chilies etc. amla is a very good source of vitamin C.
The functions of vitamin C are as follows:-
1) It is necessary for keeping teeth, gums and joints healthy.
2) It plays an important role in normal metabolism of the amino acids.
3) It helps in healing of cuts and wounds and gives resistance to our body against diseases and infections.
Now considering the statement as;
The structure of vitamin C is as;-

So, thus from the structure of Vitamin C we can see that the functional groups present in it are:- -OH group (i.e. hydroxyl group), -C=O (carbonyl group) and -O- group (i.e. ether group).
Hence, the functional group in vitamin C are hydroxyl, carbonyl and ether respectively.
Note:
Vitamin C is soluble in water and is therefore known as the water-soluble vitamins. Water soluble vitamins are those vitamins which must be supplied in the diet regularly because they are readily excreted in urine and cannot be stored in our body.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




