
What are $sp$, $s{p^2}$ and $s{p^3}$ orbitals?
Answer
495.6k+ views
Hint: The concept of intermixing of two or more atomic orbitals of different shape and nearly of same energy to generate the orbitals of same shape, equal energy and orientation as to minimize the repulsions between orbitals is known as hybridization.
Complete answer:
The reason to introduce the concept of hybridization was the failure of valence bond theory in certain aspects which are as follows:
1. Valencies of certain elements- The maximum number of covalent bonds which an atom is capable to form is equal to the number of unpaired electrons present in its valence shell. However, the valence bond theory failed to explain the covalent bonding in beryllium, boron and carbon.
2. The valence bond theory cannot explain shapes, geometries and bond angles in certain molecules like the tetrahedral shape of a methane molecule.
Thus, the concept of hybridization was introduced to overcome these limitations. The atomic orbitals formed by the result of hybridization are known as hybrid orbitals. Let’s now discuss the given hybrid orbitals.
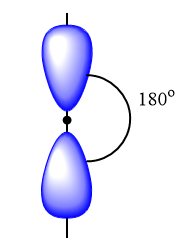
$sp$ hybrid orbitals: It is observed when one s and one p orbital of an atom mix to form two new similar orbitals. It forms linear molecules and the angle between the bond is ${180^o}$. Example: $Be{F_2}$
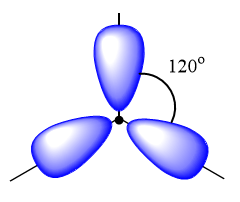
$s{p^2}$ hybrid orbitals: It is observed when one s and two p orbitals of an atom mix to three two new similar orbitals. It forms trigonal planar geometry and the angle between the bond is ${120^o}$. Example: ${C_2}{H_4}$
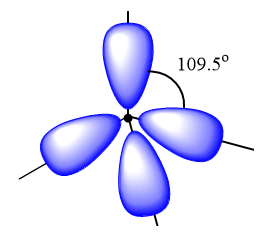
$s{p^3}$ hybrid orbitals: It is observed when one s and three p orbital of an atom mix to form four new similar orbitals. It forms molecules with tetrahedral geometry and the angle between the bond is ${109.5^o}$. Example: $C{H_4}$.
Note:
Remember that the hybrid orbitals are usually involved in the sigma bonds of polyatomic molecules whereas the pi bonds are generally formed by the overlap of unhybridized orbitals. It is important to note that the hybrid orbitals do not exist in an isolated atom.
Complete answer:
The reason to introduce the concept of hybridization was the failure of valence bond theory in certain aspects which are as follows:
1. Valencies of certain elements- The maximum number of covalent bonds which an atom is capable to form is equal to the number of unpaired electrons present in its valence shell. However, the valence bond theory failed to explain the covalent bonding in beryllium, boron and carbon.
2. The valence bond theory cannot explain shapes, geometries and bond angles in certain molecules like the tetrahedral shape of a methane molecule.
Thus, the concept of hybridization was introduced to overcome these limitations. The atomic orbitals formed by the result of hybridization are known as hybrid orbitals. Let’s now discuss the given hybrid orbitals.
$sp$ hybrid orbitals: It is observed when one s and one p orbital of an atom mix to form two new similar orbitals. It forms linear molecules and the angle between the bond is ${180^o}$. Example: $Be{F_2}$

$s{p^2}$ hybrid orbitals: It is observed when one s and two p orbitals of an atom mix to three two new similar orbitals. It forms trigonal planar geometry and the angle between the bond is ${120^o}$. Example: ${C_2}{H_4}$

$s{p^3}$ hybrid orbitals: It is observed when one s and three p orbital of an atom mix to form four new similar orbitals. It forms molecules with tetrahedral geometry and the angle between the bond is ${109.5^o}$. Example: $C{H_4}$.

Note:
Remember that the hybrid orbitals are usually involved in the sigma bonds of polyatomic molecules whereas the pi bonds are generally formed by the overlap of unhybridized orbitals. It is important to note that the hybrid orbitals do not exist in an isolated atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




