
What are exceptions to octet rule?
A.The incomplete octet of the central atom.
B.An odd no. of electrons on central atoms.
C.Expanded octet of central atom.
D.All of these.
Answer
585.3k+ views
Hint:According to octet rule, the atoms tend to form compounds in ways that give them eight valence electrons and hence, they should obey electronic configuration of noble gas.
Complete step by step answer:
General exception of octet rule includes that the molecules that have an odd number of electrons on the central atom or the molecules which have more or fewer electrons than eight in its valence shell.
Compounds which have more than an octet of electrons are known to be expanded- valence molecules.
If we see in s and p blocks, there are very less number of molecules which have odd no. of electrons but in d and f block elements it is more common.
There are examples of molecules having odd numbers of electrons like \[NO\], Molecules that contain d block elements mostly contain odd numbers of electrons and hence their bonding cannot be explained by the simple approach.
There are only a few elements present in the p block that contain an odd number of electrons.
There are examples of some molecules which have more electrons than eight in its valence shell like $S{F_6}$ , if we see the Lewis structure of $S{F_6}$ it must accommodate a total of 48 valence electrons.
Molecules with atoms that possess less than an octet of electrons generally contain the lighter s and p block elements like beryllium which have four electrons around the central atom and also, boron which have six. Example of this is $BC{l_3}$, In this case a boron atom has six valence electrons while chlorine has eight.
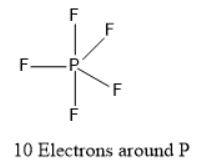


Therefore, Option D All of these exceptions is correct.
Note:
According to the octet rule, the central atom must contain eight electrons but in some compounds there are fewer or more than the eight electrons and also, an odd number of electrons present in the central atom.
Complete step by step answer:
General exception of octet rule includes that the molecules that have an odd number of electrons on the central atom or the molecules which have more or fewer electrons than eight in its valence shell.
Compounds which have more than an octet of electrons are known to be expanded- valence molecules.
If we see in s and p blocks, there are very less number of molecules which have odd no. of electrons but in d and f block elements it is more common.
There are examples of molecules having odd numbers of electrons like \[NO\], Molecules that contain d block elements mostly contain odd numbers of electrons and hence their bonding cannot be explained by the simple approach.
There are only a few elements present in the p block that contain an odd number of electrons.
There are examples of some molecules which have more electrons than eight in its valence shell like $S{F_6}$ , if we see the Lewis structure of $S{F_6}$ it must accommodate a total of 48 valence electrons.
Molecules with atoms that possess less than an octet of electrons generally contain the lighter s and p block elements like beryllium which have four electrons around the central atom and also, boron which have six. Example of this is $BC{l_3}$, In this case a boron atom has six valence electrons while chlorine has eight.



Therefore, Option D All of these exceptions is correct.
Note:
According to the octet rule, the central atom must contain eight electrons but in some compounds there are fewer or more than the eight electrons and also, an odd number of electrons present in the central atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




