
What are acetals and hemiacetals?
Answer
587.1k+ views
Hint: An acetal forms when two ether groups are attached to the same carbon. It is also prepared from a hemiacetal, which is derived from an aldehyde that contain one ether group and one alcohol group.
Complete step-by-step answer:
When aldehyde and ketones react with alcohols under an acidic environment, they form acetals. These are functional groups with tetrahedral geometry, in which two alkoxy \[\left( { - OR} \right)\] groups are bonded to the central carbon atom.
The mechanism starts with protonation of carbonyl oxygen that makes \[C = O\] bond highly electrophilic, which is then attacked by alcohol that forms oxonium intermediate. This intermediate gets deprotonated and forms a hemiacetal.
Hemiacetal has only one alkoxy group compared to acetals which has two alkoxy groups. Further, the OH group of hemiacetal is protonated and changed into a good leaving group. As carbon becomes sterically hindered, \[{S_N}2\] reaction cannot occur here.
Therefore, \[{S_N}2\] mechanism is followed here where the leaving group is first eliminated by oxygen lone pairs followed by deprotonation of oxonium intermediate that results in acetal.
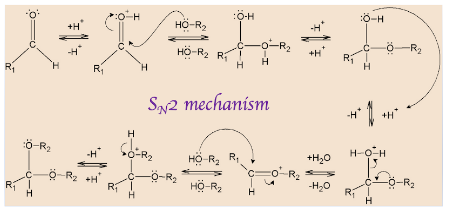
Hemiacetals are formed from an aldehyde or ketone and an alcohol, by forming an ether bond and OH bond on the same carbon. It is a tetrahedral intermediate in the formation of acetal. When it is catalysed by an acid, it becomes faster but acid can proceed it to decomposition also, back to the starting material.
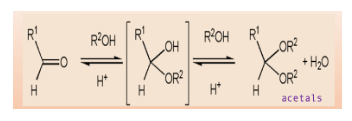
Note: A hemiacetal structure acts as a reducing sugar and thus it is unstable in nature. On the contrary, acetals are non-reducing sugars and are stable as they are not converted back into aldehyde or hemiacetals.
Complete step-by-step answer:
When aldehyde and ketones react with alcohols under an acidic environment, they form acetals. These are functional groups with tetrahedral geometry, in which two alkoxy \[\left( { - OR} \right)\] groups are bonded to the central carbon atom.
The mechanism starts with protonation of carbonyl oxygen that makes \[C = O\] bond highly electrophilic, which is then attacked by alcohol that forms oxonium intermediate. This intermediate gets deprotonated and forms a hemiacetal.
Hemiacetal has only one alkoxy group compared to acetals which has two alkoxy groups. Further, the OH group of hemiacetal is protonated and changed into a good leaving group. As carbon becomes sterically hindered, \[{S_N}2\] reaction cannot occur here.
Therefore, \[{S_N}2\] mechanism is followed here where the leaving group is first eliminated by oxygen lone pairs followed by deprotonation of oxonium intermediate that results in acetal.

Hemiacetals are formed from an aldehyde or ketone and an alcohol, by forming an ether bond and OH bond on the same carbon. It is a tetrahedral intermediate in the formation of acetal. When it is catalysed by an acid, it becomes faster but acid can proceed it to decomposition also, back to the starting material.

Note: A hemiacetal structure acts as a reducing sugar and thus it is unstable in nature. On the contrary, acetals are non-reducing sugars and are stable as they are not converted back into aldehyde or hemiacetals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




