
Aniline reacts with excess \[{\text{B}}{{\text{r}}_2}/{{\text{H}}_2}{\text{O}}\] to give the major product:
A.
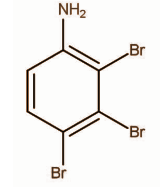
B.
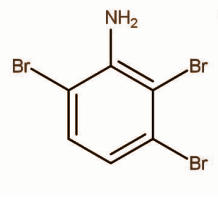
C.
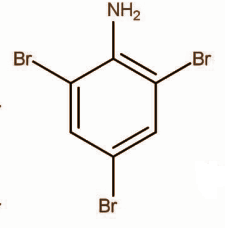
D.
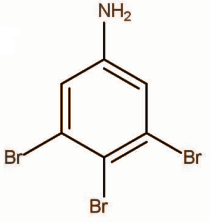




Answer
572.1k+ views
Hint: The \[{\text{N}}{{\text{H}}_2}\]group is a very good activating group. It increases the electron density at ortho and para position. Due to strong activation of benzene ring poly substitution occurs.
Complete step by step solution:
Halogenation of phenols or aniline occurs via electrophilic substitution reaction. In general to produce electrophile a Lewis acid is required. In the above reaction water acts as a Lewis acid which gives rise to the required electrophile. Water is a highly polar solvent hence it supports the poly substituted. However in the presence of low polar solvents such as chloroform and carbon tetrachloride only mono substituted product forms, ortho and para substituted products are formed.
In phenol and aniline hydroxide and amine respectively are activating groups in such cases Lewis acid are not required. Halogen which generally exists as a diatomic molecule breaks heterolytically and electrophile is generated. The reaction of aniline with bromine in the presence of water occurs as:
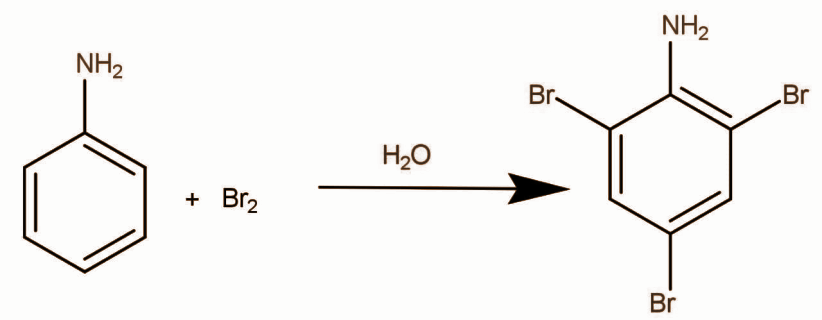
White precipitate of 2,4,6-tribromoaniline is formed.
Hence, the correct option is C.
Note: The groups like \[{\text{N}}{{\text{H}}_2}\] and \[{\text{OH}}\] show mesomeric effect. Mesomeric effect is the resonance shown by the presence of a functional group. Due to resonance these groups donate electron density to the benzene ring. The electron density increases on ortho and para position on the benzene ring. Ortho position is the position next to the functional group attached to the benzene ring. Meta position is the second position corresponding to the group attached to the functional group. Para position is the third position with respect to the functional group attached. There are 2 ortho and 2 Meta positions and only one para position.
Complete step by step solution:
Halogenation of phenols or aniline occurs via electrophilic substitution reaction. In general to produce electrophile a Lewis acid is required. In the above reaction water acts as a Lewis acid which gives rise to the required electrophile. Water is a highly polar solvent hence it supports the poly substituted. However in the presence of low polar solvents such as chloroform and carbon tetrachloride only mono substituted product forms, ortho and para substituted products are formed.
In phenol and aniline hydroxide and amine respectively are activating groups in such cases Lewis acid are not required. Halogen which generally exists as a diatomic molecule breaks heterolytically and electrophile is generated. The reaction of aniline with bromine in the presence of water occurs as:

White precipitate of 2,4,6-tribromoaniline is formed.
Hence, the correct option is C.
Note: The groups like \[{\text{N}}{{\text{H}}_2}\] and \[{\text{OH}}\] show mesomeric effect. Mesomeric effect is the resonance shown by the presence of a functional group. Due to resonance these groups donate electron density to the benzene ring. The electron density increases on ortho and para position on the benzene ring. Ortho position is the position next to the functional group attached to the benzene ring. Meta position is the second position corresponding to the group attached to the functional group. Para position is the third position with respect to the functional group attached. There are 2 ortho and 2 Meta positions and only one para position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




