
Aniline reacts with conc. \[\;{\text{HN}}{{\text{O}}_{\text{3}}}\] to give:-
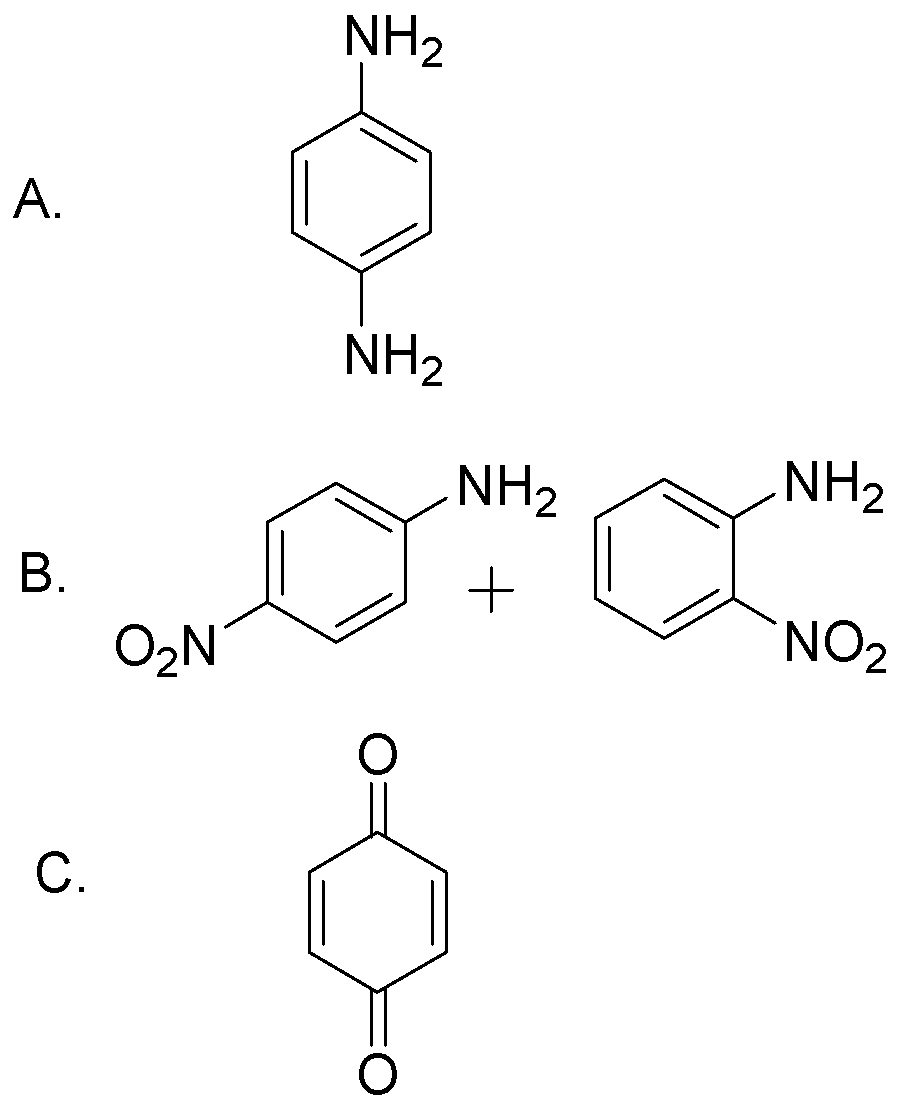

Answer
591k+ views
Hint: We must remember the reactivity of aniline. If we react to aniline with conc. \[\;{\text{HN}}{{\text{O}}_{\text{3}}}\] we will get benzoquinone. Because concentrate nitric acid is a strong oxidising agent which oxidises the amine group into carbonyl compounds in benzene rings.
Complete step by step answer:
In the presence of concentrated nitric acid, aniline gets oxidised to give p-benzoquinone.
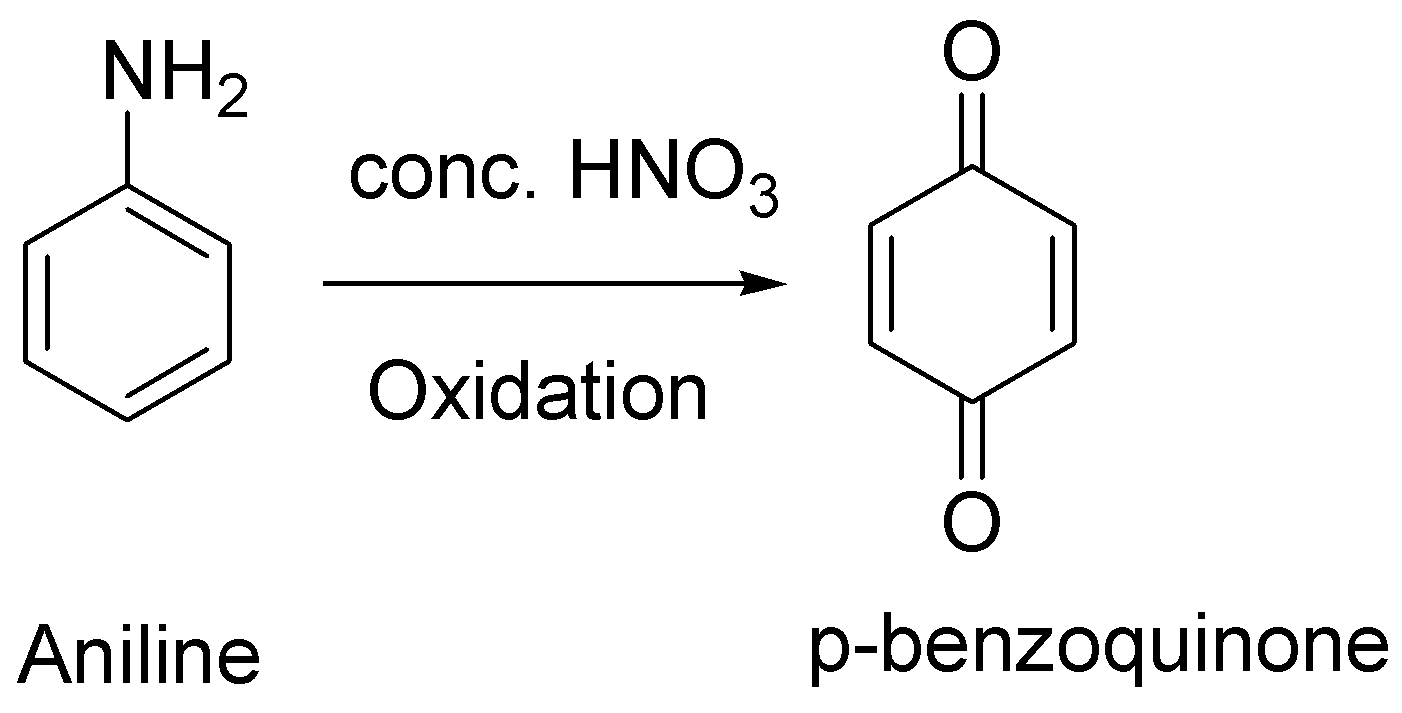
Generally in the presence of nitric acid and sulphuric acid, aniline forms anilinium nitrate as an intermediate product and forms azobenzene nitrate.
Hence, option C is the correct answer.
Additional Information:-
1. Aniline is a light brownish oily liquid with a musty and smells like the odour of a rotten fish. It is a flammable liquid and has an unpleasant odour. The compound is soluble in water. Aniline has a boiling point of $184^oC$ and melting point of about $-6^oC$ .
2. Basicity of aniline
Aniline is a weak base. They react with strong acids to form anilinium (or phenylammonium) ion\[\left( {{C_6}{H_5} - N{H_3}^ + } \right)\].
Therefore, aniline when treated with a mixture of sulphuric acid and nitric acid gives a mixture of o-, m- and p- nitroaniline.
3. Since aniline is a base, it is used to make dyes, drugs, explosives, plastics, and photographic and rubber chemicals. It is used in the large-scale production of drugs such as paracetamol, Tylenol, acetaminophen. It is also used as a pesticide and fungicides in the agricultural industry.
Note:
1. Instead of conc. nitric acid, a mixture of sulphuric acid and potassium dichromate can also be used to oxidise aniline into p-benzoquinone
2. Benzoquinone is used as a hydrogen acceptor and an oxidant in much important organic synthesis. 1,4-Benzoquinone is employed as a dehydrogenation reagent. It also serves as a dienophile in Diels Alder reactions. It is also used to suppress double-bond migration during olefin metathesis reactions.
Complete step by step answer:
In the presence of concentrated nitric acid, aniline gets oxidised to give p-benzoquinone.

Generally in the presence of nitric acid and sulphuric acid, aniline forms anilinium nitrate as an intermediate product and forms azobenzene nitrate.
Hence, option C is the correct answer.
Additional Information:-
1. Aniline is a light brownish oily liquid with a musty and smells like the odour of a rotten fish. It is a flammable liquid and has an unpleasant odour. The compound is soluble in water. Aniline has a boiling point of $184^oC$ and melting point of about $-6^oC$ .
2. Basicity of aniline
Aniline is a weak base. They react with strong acids to form anilinium (or phenylammonium) ion\[\left( {{C_6}{H_5} - N{H_3}^ + } \right)\].
Therefore, aniline when treated with a mixture of sulphuric acid and nitric acid gives a mixture of o-, m- and p- nitroaniline.
3. Since aniline is a base, it is used to make dyes, drugs, explosives, plastics, and photographic and rubber chemicals. It is used in the large-scale production of drugs such as paracetamol, Tylenol, acetaminophen. It is also used as a pesticide and fungicides in the agricultural industry.
Note:
1. Instead of conc. nitric acid, a mixture of sulphuric acid and potassium dichromate can also be used to oxidise aniline into p-benzoquinone
2. Benzoquinone is used as a hydrogen acceptor and an oxidant in much important organic synthesis. 1,4-Benzoquinone is employed as a dehydrogenation reagent. It also serves as a dienophile in Diels Alder reactions. It is also used to suppress double-bond migration during olefin metathesis reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




