
Why is aniline less basic than methylamine?
Answer
586.8k+ views
Hint: According to the concept of Lewis acid-base theory the compound or molecule that can accept hydrogen ion is known as base. Higher the tendency to accept hydrogen ion higher will be the basicity of that compound. Basic character increases with increase in donation of lone pair of electrons.
Complete step by step answer:
Aniline is an aromatic amine. The basicity of the aromatic amine is depending upon the availability of the lone pair. The higher the lone pair availability, the higher the donating ability of the lone pair as well as the accepted tendency of hydrogen ions.
In the case of aromatic amine, the lone pair undergoes conjugation with the benzene. As a result, lone pair availability decreases as well as the basicity. The resonance is shown below.
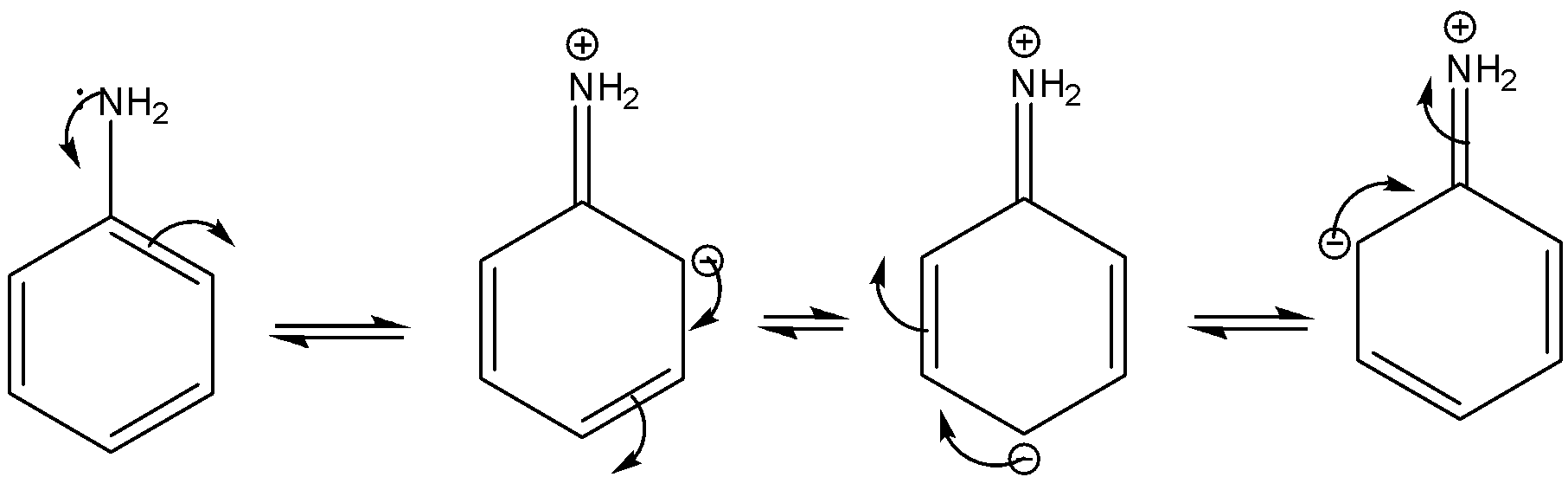
On the other hand, methylamine is an aliphatic amine. In the case of the aliphatic amine with increasing the inductive effect of the alkyl group the electron density of the nitrogen increases as well as the basicity.
In case of aniline due to conjugation the lone pair density is less than that of methylamine. Due to this reason, aniline is less basic than methylamine.
Note:
The acidity of an organic compound is depending upon the electron deficiency of the hydrogen atom. The higher the electron deficiency of the hydrogen higher will be the acidity character of that hydrogen.
Now to be acidic hydrogen that hydrogen should be attached with a high electronegative group or electron-withdrawing group. The higher the electronegativity of the group, the higher will be the electron deficiency of the hydrogen, attached to that group as well as the acidity.
Complete step by step answer:
Aniline is an aromatic amine. The basicity of the aromatic amine is depending upon the availability of the lone pair. The higher the lone pair availability, the higher the donating ability of the lone pair as well as the accepted tendency of hydrogen ions.
In the case of aromatic amine, the lone pair undergoes conjugation with the benzene. As a result, lone pair availability decreases as well as the basicity. The resonance is shown below.

On the other hand, methylamine is an aliphatic amine. In the case of the aliphatic amine with increasing the inductive effect of the alkyl group the electron density of the nitrogen increases as well as the basicity.
In case of aniline due to conjugation the lone pair density is less than that of methylamine. Due to this reason, aniline is less basic than methylamine.
Note:
The acidity of an organic compound is depending upon the electron deficiency of the hydrogen atom. The higher the electron deficiency of the hydrogen higher will be the acidity character of that hydrogen.
Now to be acidic hydrogen that hydrogen should be attached with a high electronegative group or electron-withdrawing group. The higher the electronegativity of the group, the higher will be the electron deficiency of the hydrogen, attached to that group as well as the acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




