
Angle between p-orbitals is:
A.${{90}^{0}}$
B.${{180}^{0}}$
C.${{120}^{0}}$
D.${{109}^{0}}28'$
Answer
578.4k+ views
Hint: The p orbitals are placed in such a way that electronic repulsion is minimized. The lobes at the front face away from each other, forming a straight line. P-orbitals are generally dumb-bell shaped.
Complete step-by step solution:
-In order to answer our question, let us know some facts about the p-orbitals.
-Shape: For p-orbitals, l=1, so there are three possible orientations as m= -1/0/1. This means that there are three p-orbitals whose axes are mutually perpendicular. These are designated as ${{p}_{x}},{{p}_{y}},{{p}_{z}}$ in each p subshells. The three orbitals are equal in size, shape and energy, but their orientation is different.
-Size and energy: P-orbitals increase in size and energy with the increase in principal quantum number. The order is: $4p>3p>2p$
Nodes:
-Radial nodes: Radial nodes are circular rings that occur as the principal quantum number increases. Here, the number of radial nodes for p orbitals is given by (n-2) formula. Thus there is no radial node in 2p orbital or number of radial nodes also calculated by the formula= n-l-1.
-Angular nodes: Angular nodes represent the plane/ planes that pass through the nucleus, or the origin where the probability density is equal to zero. Angular nodes number is equal to the value of ‘l’.
Following are the three p-orbitals:
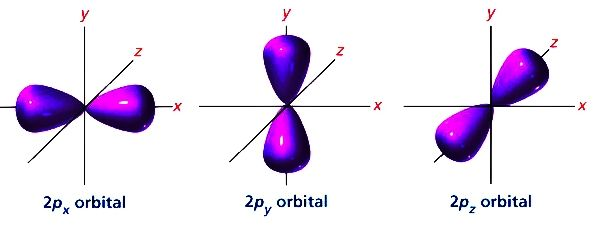
So, we can clearly see from the diagram that the orbitals are aligned according to their respective axes. Since the x,y and z axes are aligned perpendicular to each other, so two p-orbitals are also aligned perpendicular to each other.
So, we get our answer as option A.
NOTE: Following is the table which gives an idea about the number of nodes in p orbital:
Complete step-by step solution:
-In order to answer our question, let us know some facts about the p-orbitals.
-Shape: For p-orbitals, l=1, so there are three possible orientations as m= -1/0/1. This means that there are three p-orbitals whose axes are mutually perpendicular. These are designated as ${{p}_{x}},{{p}_{y}},{{p}_{z}}$ in each p subshells. The three orbitals are equal in size, shape and energy, but their orientation is different.
-Size and energy: P-orbitals increase in size and energy with the increase in principal quantum number. The order is: $4p>3p>2p$
Nodes:
-Radial nodes: Radial nodes are circular rings that occur as the principal quantum number increases. Here, the number of radial nodes for p orbitals is given by (n-2) formula. Thus there is no radial node in 2p orbital or number of radial nodes also calculated by the formula= n-l-1.
-Angular nodes: Angular nodes represent the plane/ planes that pass through the nucleus, or the origin where the probability density is equal to zero. Angular nodes number is equal to the value of ‘l’.
Following are the three p-orbitals:

So, we can clearly see from the diagram that the orbitals are aligned according to their respective axes. Since the x,y and z axes are aligned perpendicular to each other, so two p-orbitals are also aligned perpendicular to each other.
So, we get our answer as option A.
NOTE: Following is the table which gives an idea about the number of nodes in p orbital:
| Orbital | Radial nodes |
| 2p | 0 |
| 3p | 1 |
| 4p | 2. |
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




