
An unknown compound \[(A)\]has a molecular formula \[{C_4}{H_6}.\]When \[(A)\]is treated with excess of \[B{r_2}\] a new substance\[(B)\] with formula \[{C_4}{H_6}B{r_4}\] is formed. \[(A)\] forms a white ppt. with ammoniacal silver nitrate solution.\[(A)\] may be:
A. But-1-yne
B. But-2-yne
C. But-1-ene
D. But-2-ene
Answer
578.1k+ views
Hint: \[{C_4}{H_6}\] is alkyne. Alkynes are unsaturated hydrocarbons having triple bonds between at least two adjacent carbon atoms.
It’s general formula is \[{C_n}{H_{2n - 2}}\]
Complete step by step answer:
Let us form an equation according to the given condition
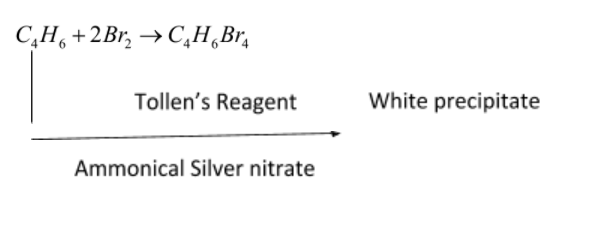
\[{C_4}{H_6}\]is butyne. It can either be But-1-yne or But-2-yne.
\[C{H_3} - C{H_2} - C \equiv CH\] But-1-yne
\[C{H_3} - C \equiv C - C{H_3}\] But-2-yne
If the triple bond is present with the carbon atom \[1\] and \[2\], then the hydrogen atom is present at C-atom \[1\] so it is acidic in nature.
\[C{H_3} - C{H_2} - C \equiv CH\]is acidic in nature.
Since Ammoniacal silver nitrate is reducing agent, it reduces butyne, and butyne oxidize ammonical silver nitrate solution.
We know that oxidation and Reduction occur together.
But-1-yne reduces by tollen’s reagent and form white precipitate
\[C{H_3} - C{H_2} - C \equiv {C^ - }{H^ + }\]
So, the correct answer is “Option A”.
Additional Information:
Tollen’s Reagent is an ammoniacal solution of \[AgN{O_3}\]. It is prepared by adding \[N{H_4}OH\] solution to\[AgN{O_3}\] solution till the precipitate of \[A{g_2}O\] is formed which just gets dissolved. It has the formula \[{[Ag{(N{O_3})_2}]^ + }O{H^{^ - }}\]
$2AgN{O_3} + 2N{H_4}OH \to A{g_2}O + 2N{H_4}N{O_3} + {H_2}O $
$A{g_2}O + 4N{H_4}OH \to 2{[Ag{(N{H_3})_2}]^ + }O{H^ - } + {H_2}O $
We can define oxidation and reduction as follows:
Addition of oxygen or removal of hydrogen is called Oxidation.
Addition of hydrogen or removal of oxygen is called Reduction.
Since But-1-yne is acidic in nature. So it can easily remove H-atoms and oxidates, while Tollen’s reagent reduces. But-2-yne does not have acidic hydrogen, so it will not react with tollen’s reagent.
Note:
Oxidation and reduction occurs simultaneously. This reaction is a Redox reaction in which But-1-yne reduces and Tollen’s reagent oxidises.
It’s general formula is \[{C_n}{H_{2n - 2}}\]
Complete step by step answer:
Let us form an equation according to the given condition

\[{C_4}{H_6}\]is butyne. It can either be But-1-yne or But-2-yne.
\[C{H_3} - C{H_2} - C \equiv CH\] But-1-yne
\[C{H_3} - C \equiv C - C{H_3}\] But-2-yne
If the triple bond is present with the carbon atom \[1\] and \[2\], then the hydrogen atom is present at C-atom \[1\] so it is acidic in nature.
\[C{H_3} - C{H_2} - C \equiv CH\]is acidic in nature.
Since Ammoniacal silver nitrate is reducing agent, it reduces butyne, and butyne oxidize ammonical silver nitrate solution.
We know that oxidation and Reduction occur together.
But-1-yne reduces by tollen’s reagent and form white precipitate
\[C{H_3} - C{H_2} - C \equiv {C^ - }{H^ + }\]
So, the correct answer is “Option A”.
Additional Information:
Tollen’s Reagent is an ammoniacal solution of \[AgN{O_3}\]. It is prepared by adding \[N{H_4}OH\] solution to\[AgN{O_3}\] solution till the precipitate of \[A{g_2}O\] is formed which just gets dissolved. It has the formula \[{[Ag{(N{O_3})_2}]^ + }O{H^{^ - }}\]
$2AgN{O_3} + 2N{H_4}OH \to A{g_2}O + 2N{H_4}N{O_3} + {H_2}O $
$A{g_2}O + 4N{H_4}OH \to 2{[Ag{(N{H_3})_2}]^ + }O{H^ - } + {H_2}O $
We can define oxidation and reduction as follows:
Addition of oxygen or removal of hydrogen is called Oxidation.
Addition of hydrogen or removal of oxygen is called Reduction.
Since But-1-yne is acidic in nature. So it can easily remove H-atoms and oxidates, while Tollen’s reagent reduces. But-2-yne does not have acidic hydrogen, so it will not react with tollen’s reagent.
Note:
Oxidation and reduction occurs simultaneously. This reaction is a Redox reaction in which But-1-yne reduces and Tollen’s reagent oxidises.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




