
An organic compound which produces a bluish-green coloured flame on heating in presence of copper is:
A. Chlorobenzene
B. Benzaldehyde
C. Aniline
D. Benzoic acid
Answer
578.4k+ views
Hint: The organic compound that has halogen gives a positive result for Beilstein’s test. The bluish-green coloured flame indicates the presence of halogen in the organic compound.
Complete answer:
The organic compound that has halogen gives the bluish-green coloured flame during Beilstein’s test.
The organic compound is heated on the copper wire on the burner. As the organic compound burns it decomposes. The halogen of organic compounds reacts with copper and forms copper halide and produces a bluish-green coloured flame.
The structures of all the compounds are as follows:
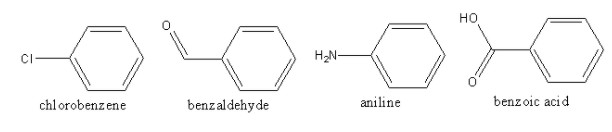
In chlorobenzene, chlorine is attached with a benzene ring, so chlorobenzene can produce bluish-green coloured flame on reacting with copper, so option (A) is correct.
In benzaldehyde, an aldehyde group is attached with a benzene ring so, benzaldehyde does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (B) is incorrect.
In aniline, ammine is attached with a benzene ring so aniline does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (C) is incorrect.
In benzoic acid, carboxylic groups are attached with benzene rings so benzoic acid does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (D) is incorrect.
Therefore option (A) chlorobenzene, is correct.
Note: Beilstein’s test is used for the detection of halogens in the organic compound. The bluish-green coloured flame indicates the formation of copper halide. Beilstein’s test does not determine the presence of fluorine.
Complete answer:
The organic compound that has halogen gives the bluish-green coloured flame during Beilstein’s test.
The organic compound is heated on the copper wire on the burner. As the organic compound burns it decomposes. The halogen of organic compounds reacts with copper and forms copper halide and produces a bluish-green coloured flame.
The structures of all the compounds are as follows:

In chlorobenzene, chlorine is attached with a benzene ring, so chlorobenzene can produce bluish-green coloured flame on reacting with copper, so option (A) is correct.
In benzaldehyde, an aldehyde group is attached with a benzene ring so, benzaldehyde does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (B) is incorrect.
In aniline, ammine is attached with a benzene ring so aniline does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (C) is incorrect.
In benzoic acid, carboxylic groups are attached with benzene rings so benzoic acid does not have halogen, so it will not produce a bluish-green coloured flame on reacting with copper, so option (D) is incorrect.
Therefore option (A) chlorobenzene, is correct.
Note: Beilstein’s test is used for the detection of halogens in the organic compound. The bluish-green coloured flame indicates the formation of copper halide. Beilstein’s test does not determine the presence of fluorine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




