
An organic compound ‘A’ on treatment with ammoniacal silver nitrate gives metallic silver and produces a yellow crystalline precipitate of molecular formula ${{C}_{9}}{{H}_{10}}{{N}_{4}}{{O}_{4}}$ on treatment with Brady’s reagent. Give the structure of the organic compound ‘A’.
Answer
584.7k+ views
Hint: Ammoniacal silver nitrate is known as Tollen’s reagent and it is used to detect the aldehydes group. If compound ‘A’ reacts with Tollen’s reagent then it must be aldehydes. The Brady's reagent has 6 carbon atoms by subtracting the number of carbon atoms from the precipitate formed, the compound 'A' can be detected.
Complete step by step solution:
An organic compound ‘A’ on treatment with ammoniacal silver nitrate gives metallic silver: Ammoniacal silver nitrate is known as Tollen’s reagent and it is used to detect the aldehydes group. When $N{{H}_{4}}OH$ solution is added to $AgN{{O}_{3}}$ solution until the ppt. of $A{{g}_{2}}O$ first formed just redissolves. When an aldehyde is reacted with Tollen’s reagent the later is reduced to metallic silver. The reaction is given below:
$RCHO+2{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}}+3O{{H}^{-}}\to RCC{{O}^{-}}+2Ag+4N{{H}_{3}}+2{{H}_{2}}O$
The question says that when it is reacted with Brady’s reagent it forms ${{C}_{9}}{{H}_{10}}{{N}_{4}}{{O}_{4}}$. Brady’s reagent is chemically known as 2,4-dinitrophenylhydrazine. The formula of 2,4-dinitrophenylhydrazine is ${{C}_{6}}{{H}_{6}}{{N}_{4}}{{O}_{4}}$. The structure of 2,4-dinitrophenylhydrazine is:
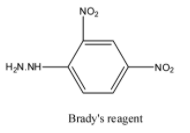
If an aldehyde reacts with Brady’s reagent, a yellow, orange, or red precipitate is formed. The aldehyde gets attached to Brady's reagent by the removal of the water molecule. Since the compound formed is ${{C}_{9}}{{H}_{10}}{{N}_{4}}{{O}_{4}}$, it has three carbon atom more than Brady's reagent. So, the compound is propanol. The reaction is:
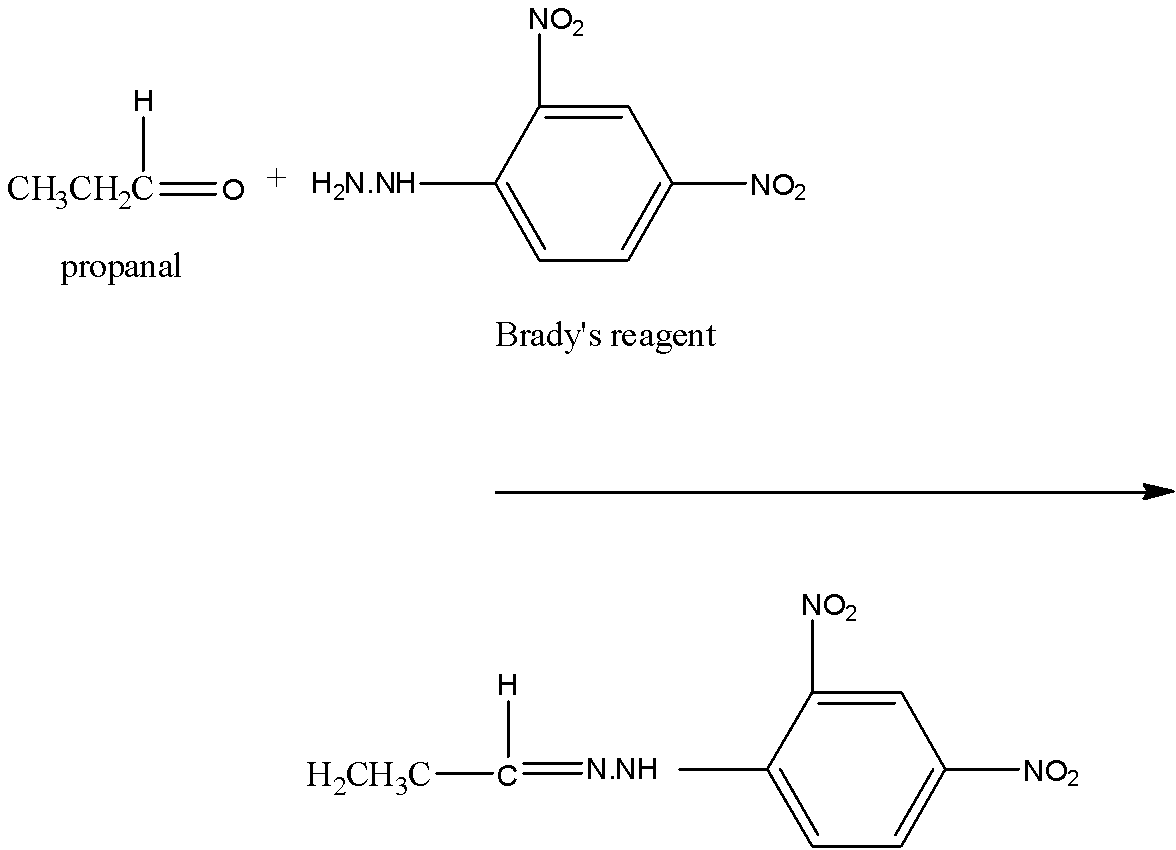
Note: Tollen’s reagent is used to identify the aldehydes group in the compound, it must be noted that both aliphatic and aromatic aldehydes give the Tollen’s test. It is also known as the silver mirror test.
Complete step by step solution:
An organic compound ‘A’ on treatment with ammoniacal silver nitrate gives metallic silver: Ammoniacal silver nitrate is known as Tollen’s reagent and it is used to detect the aldehydes group. When $N{{H}_{4}}OH$ solution is added to $AgN{{O}_{3}}$ solution until the ppt. of $A{{g}_{2}}O$ first formed just redissolves. When an aldehyde is reacted with Tollen’s reagent the later is reduced to metallic silver. The reaction is given below:
$RCHO+2{{[Ag{{(N{{H}_{3}})}_{2}}]}^{+}}+3O{{H}^{-}}\to RCC{{O}^{-}}+2Ag+4N{{H}_{3}}+2{{H}_{2}}O$
The question says that when it is reacted with Brady’s reagent it forms ${{C}_{9}}{{H}_{10}}{{N}_{4}}{{O}_{4}}$. Brady’s reagent is chemically known as 2,4-dinitrophenylhydrazine. The formula of 2,4-dinitrophenylhydrazine is ${{C}_{6}}{{H}_{6}}{{N}_{4}}{{O}_{4}}$. The structure of 2,4-dinitrophenylhydrazine is:

If an aldehyde reacts with Brady’s reagent, a yellow, orange, or red precipitate is formed. The aldehyde gets attached to Brady's reagent by the removal of the water molecule. Since the compound formed is ${{C}_{9}}{{H}_{10}}{{N}_{4}}{{O}_{4}}$, it has three carbon atom more than Brady's reagent. So, the compound is propanol. The reaction is:

Note: Tollen’s reagent is used to identify the aldehydes group in the compound, it must be noted that both aliphatic and aromatic aldehydes give the Tollen’s test. It is also known as the silver mirror test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




