
An optically active amine (A) is subjected to exhaustive methylation and Hofmann elimination to yield an alkene (B). (B) on ozonolysis gives an equimolar mixture of formaldehyde and butanal. Deduce the structural of (A) and (B). Is there any structural isomer (A), if yes draw its structure?
Answer
544.5k+ views
Hint:In the above question, we have to determine the structure of A and B and we have to check if there is any structural isomer of A. Here, since the end products are given we should try to solve these questions backwardly. In short, first we have to see which product results in formation of formaldehyde and butanal and then we can proceed further. The first reaction results in formation of alkene and the second forms aldehyde.
Complete step-by-step answer:Ozonolysis is a process which takes place in unsaturated bonds of alkene or alkyne where the bond is replaced by oxygen atom.
In the above question, formaldehyde and butanal are formed, so we can replace the oxygen atom present in both the molecules by unsaturated carbon-carbon bond which indicates that the resultant reactant was pent-1-ene.
The reaction involving the above reaction is:

Pent-1-ene is formed by exhaustive methylation and Hoffmann elimination of A.
Since, in exhaustive methylation ${\text{C}}{{\text{H}}_{\text{3}}}$ group is added in place of hydrogen atom in ${\text{N}}{{\text{H}}_{\text{3}}}$ to makes nitrogen more electropositive and then Hofmann elimination takes place. It states that the less substituted alkene is the major product. Hence, the product formed is
The reaction involving the above reaction is:
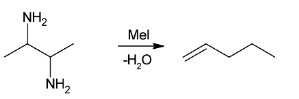
Overall reaction can be illustrated as:
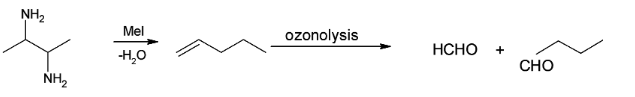
There is no structural isomer of A.
Note:Formaldehyde is a colourless and strong smelling gas which is used in building and household products.
Butanal is used in the manufacture of rubber accelerators, synthetic resins and many more.
Complete step-by-step answer:Ozonolysis is a process which takes place in unsaturated bonds of alkene or alkyne where the bond is replaced by oxygen atom.
In the above question, formaldehyde and butanal are formed, so we can replace the oxygen atom present in both the molecules by unsaturated carbon-carbon bond which indicates that the resultant reactant was pent-1-ene.
The reaction involving the above reaction is:

Pent-1-ene is formed by exhaustive methylation and Hoffmann elimination of A.
Since, in exhaustive methylation ${\text{C}}{{\text{H}}_{\text{3}}}$ group is added in place of hydrogen atom in ${\text{N}}{{\text{H}}_{\text{3}}}$ to makes nitrogen more electropositive and then Hofmann elimination takes place. It states that the less substituted alkene is the major product. Hence, the product formed is
The reaction involving the above reaction is:

Overall reaction can be illustrated as:

There is no structural isomer of A.
Note:Formaldehyde is a colourless and strong smelling gas which is used in building and household products.
Butanal is used in the manufacture of rubber accelerators, synthetic resins and many more.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




