
An ideal binary solution is prepared by two liquids A and B, with \[P_A^0 > P_B^0\] . Then:
A. A-B interactions are stronger than A-A and B-B
B. A and B have the same molecular masses
C. A-B interactions are similar to A-A and B-B interactions
D. Both A and B are non-polar substances
Answer
582.3k+ views
Hint:For an ideal gas, the enthalpy of the solution gets closer to zero and shows an ideal behavior. Also, the volume remains the same in mixing the solution. For vapor pressure, one must know Raoult’s law. The entropy always increases for an ideal gas and so the Gibbs free energy is negative.
Complete step by step answer:
Raoult’s law states that a solvent’s partial vapor pressure in a mixture or solution is identical to the vapor pressure of the pure solvent multiplied by its mole fraction. Solutions that obey this law are ideal and which do not obey are non-ideal solutions.
For an ideal solution, as per Raoult’s law, the partial vapor pressure of a solvent in a solution is equal to the vapor pressure of a pure solvent multiplied by its mole fraction.
We have \[{P_A} = {x_A}P_A^o,{P_B} = {x_B}P_B^o\]
Therefore, \[\Delta P = {P_{obs}} - {P_{calculated\,by\,Raoult's\,law}} = 0\]
Therefore, an ideal binary solution is prepared by two liquids A and B, with \[P_A^0 > P_B^0\] . Then A-B interactions are similar to A-A and B-B interactions.
So, the correct answer is C.
Additional information:
An ideal solution is a homogeneous mixture of compounds under which the interactions between the solute and solvent molecules are the same as those between the molecules itself of each substance. For example, benzene and toluene have very similar molecular structures and so they are ideal.
We know that, \[\Delta {U_{mix}} = \Delta {H_{mix}} - P\Delta {V_{mix}}\] from the first law of thermodynamics
For an ideal solution, the solution enthalpy reached approximately to zero and the volume mixing is also zero for an ideal gas. During mixing, no heat should be either evolved or absorbed and there should be no expansion or contraction during mixing.
\[\Delta {H_{mix}} = 0,\Delta {V_{mix}} = 0\]
\[\therefore \Delta {U_{mix}} = 0\]
For ideal solution, the Gibbs free energy can be written as:
\[\Delta {G_{mix}} = \Delta {H_{mix}} - T\Delta {S_{mix}}\]
\[\Delta {S_{mix}} \ne 0\] for an ideal gas
Thus, \[\Delta {G_{mix}} \ne 0\]
Note:The solutions which do not obey Raoult’s law can be volatile or non-volatile. They can either dissociate or associate in a solution. Because the solutions that obey the law have these limitations that the solution should contain non-volatile solutes only and must not associate or dissociate in solution.
For example, chloroform and acetone show a negative deviation from Raoult’s law. This is because there is an attractive interaction between these two that results in the formation of a hydrogen bonding as shown below.
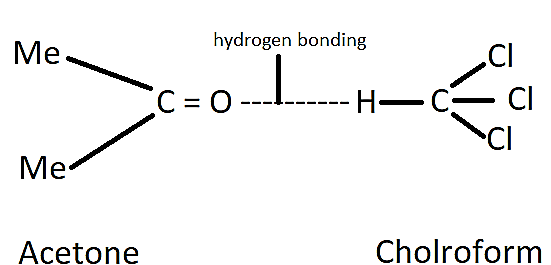
Complete step by step answer:
Raoult’s law states that a solvent’s partial vapor pressure in a mixture or solution is identical to the vapor pressure of the pure solvent multiplied by its mole fraction. Solutions that obey this law are ideal and which do not obey are non-ideal solutions.
For an ideal solution, as per Raoult’s law, the partial vapor pressure of a solvent in a solution is equal to the vapor pressure of a pure solvent multiplied by its mole fraction.
We have \[{P_A} = {x_A}P_A^o,{P_B} = {x_B}P_B^o\]
Therefore, \[\Delta P = {P_{obs}} - {P_{calculated\,by\,Raoult's\,law}} = 0\]
Therefore, an ideal binary solution is prepared by two liquids A and B, with \[P_A^0 > P_B^0\] . Then A-B interactions are similar to A-A and B-B interactions.
So, the correct answer is C.
Additional information:
An ideal solution is a homogeneous mixture of compounds under which the interactions between the solute and solvent molecules are the same as those between the molecules itself of each substance. For example, benzene and toluene have very similar molecular structures and so they are ideal.
We know that, \[\Delta {U_{mix}} = \Delta {H_{mix}} - P\Delta {V_{mix}}\] from the first law of thermodynamics
For an ideal solution, the solution enthalpy reached approximately to zero and the volume mixing is also zero for an ideal gas. During mixing, no heat should be either evolved or absorbed and there should be no expansion or contraction during mixing.
\[\Delta {H_{mix}} = 0,\Delta {V_{mix}} = 0\]
\[\therefore \Delta {U_{mix}} = 0\]
For ideal solution, the Gibbs free energy can be written as:
\[\Delta {G_{mix}} = \Delta {H_{mix}} - T\Delta {S_{mix}}\]
\[\Delta {S_{mix}} \ne 0\] for an ideal gas
Thus, \[\Delta {G_{mix}} \ne 0\]
Note:The solutions which do not obey Raoult’s law can be volatile or non-volatile. They can either dissociate or associate in a solution. Because the solutions that obey the law have these limitations that the solution should contain non-volatile solutes only and must not associate or dissociate in solution.
For example, chloroform and acetone show a negative deviation from Raoult’s law. This is because there is an attractive interaction between these two that results in the formation of a hydrogen bonding as shown below.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




