
An exothermic reaction is represented by the graph:
A.

B.

C.
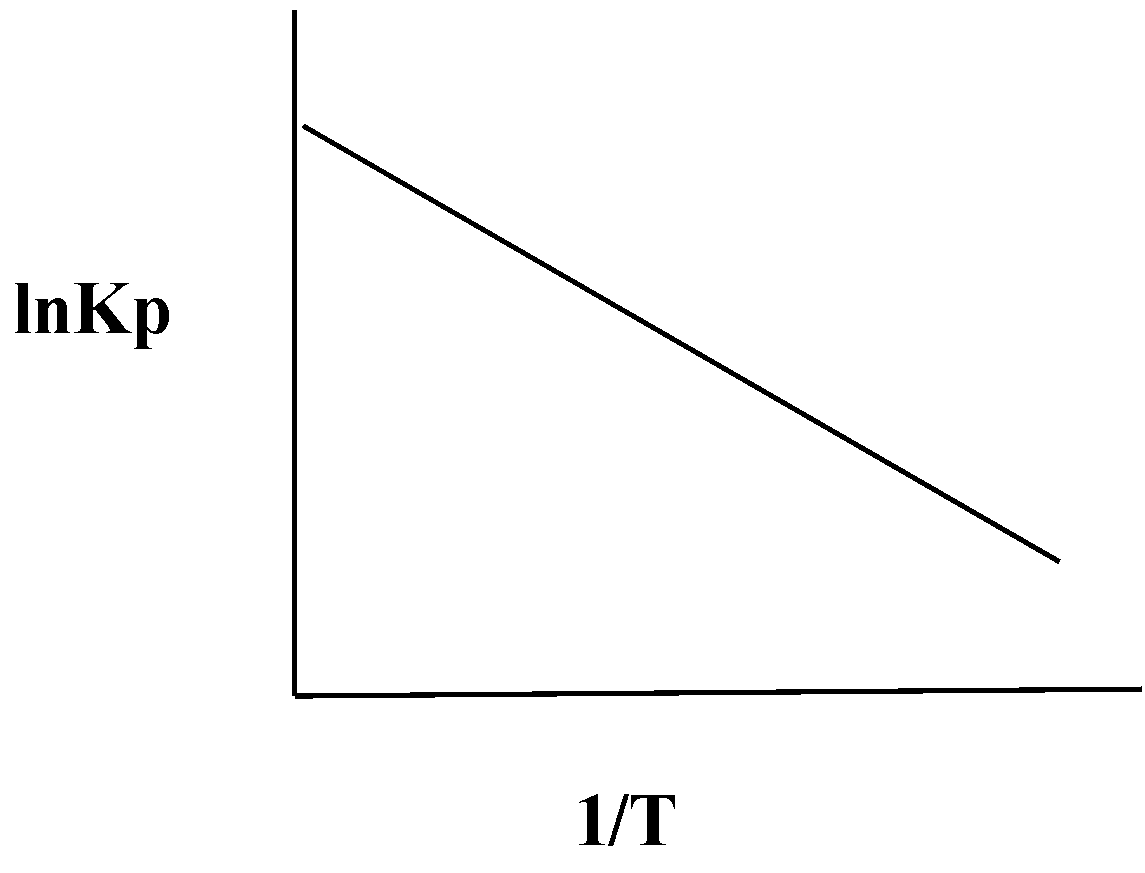
D.




Answer
522.2k+ views
Hint: The relation between equilibrium constant, ${K_p}$ and Temperature is given by Van’t Hoff’s equation. The equation relates the change in the equilibrium constant of a reaction with the change in temperature and the standard enthalpy change of a reaction. And for an exothermic reaction, the standard enthalpy change is always negative. This is because energy is released during an exothermic reaction.
Complete step by step answer:
The van’t Hoff equation was proposed by a Dutch chemist Jacobus Henricus van’t Hoff in the year 1884. The van’t Hoff’s equation is given by:
$\ln {K_p} = \dfrac{{\Delta {H^0}}}{{RT}} + \dfrac{{\Delta {S^0}}}{R}$, here $\Delta {H^0}$ is the change in standard enthalpy, R is the universal gas constant, T is the temperature, ${K_p}$ is the equilibrium constant, and $\Delta {S^0}$ is the change in the entropy.
If you look at the van’t Hoff equation it is in the form $y = mx + c$ where m is the slope of the reaction and c is the intercept.
So comparing both the equations the slope of the reaction is $ - \dfrac{{\Delta {H^0}}}{R}$ , since for an exothermic change is standard enthalpy is negative, it will give us a positive slope.
So, the correct answer is Option A.
Note: The equilibrium constant of a reaction is the value of the reaction quotient of that chemical reaction at a chemical equilibrium, which is a state approached by a dynamic chemical system after sufficient time has passed at which its composition does not change further. In simple words, an equilibrium is attained when the rate of the forward reaction is equal to the rate of backward reaction.
Complete step by step answer:
The van’t Hoff equation was proposed by a Dutch chemist Jacobus Henricus van’t Hoff in the year 1884. The van’t Hoff’s equation is given by:
$\ln {K_p} = \dfrac{{\Delta {H^0}}}{{RT}} + \dfrac{{\Delta {S^0}}}{R}$, here $\Delta {H^0}$ is the change in standard enthalpy, R is the universal gas constant, T is the temperature, ${K_p}$ is the equilibrium constant, and $\Delta {S^0}$ is the change in the entropy.
If you look at the van’t Hoff equation it is in the form $y = mx + c$ where m is the slope of the reaction and c is the intercept.
So comparing both the equations the slope of the reaction is $ - \dfrac{{\Delta {H^0}}}{R}$ , since for an exothermic change is standard enthalpy is negative, it will give us a positive slope.
So, the correct answer is Option A.
Note: The equilibrium constant of a reaction is the value of the reaction quotient of that chemical reaction at a chemical equilibrium, which is a state approached by a dynamic chemical system after sufficient time has passed at which its composition does not change further. In simple words, an equilibrium is attained when the rate of the forward reaction is equal to the rate of backward reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




