
An example of non reducing sugar is:
A. Cane sugar
B. Fructose
C. Lactose
D. Glucose
Answer
600.3k+ views
Hint: The sugars which do not have a hydroxyl group attached to the anomeric carbon are called reducing sugars. When the sugar is in the open configuration, the alcohol group becomes a ketone or an aldehyde group.
Complete step by step answer:
Any sugar which can act as a reducing agent is known as a reducing sugar. It can act as a reducing agent due to the presence of a free aldehyde group or a free ketone group. All monosaccharides are reducing sugars along with some disaccharides, some oligosaccharides, and some polysaccharides.
The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. Examples of monosaccharide reducing sugars are galactose, glucose, fructose, etc.
A disaccharide is formed from two monosaccharides. A disaccharide can be classified as a reducing sugar or a non – reducing sugar based on presence of a hydroxyl group at the anomeric carbon. If the hydroxyl group is present at the anomeric carbon, then the disaccharide is reducing, else it is a non – reducing sugar.
In the given question, sucrose is a non-reducing sugar due to the absence of hydroxyl group at the anomeric carbon. It is a dimer of glucose and fructose. It is also known as cane sugar. The structure of sucrose is:
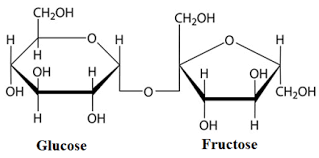
Hence, the correct answer is (A) cane sugar.
Note: Remember that a ketose sugar first tautomerizes into an aldose sugar and then it is reduced. It can’t be oxidised in ketose form.
Complete step by step answer:
Any sugar which can act as a reducing agent is known as a reducing sugar. It can act as a reducing agent due to the presence of a free aldehyde group or a free ketone group. All monosaccharides are reducing sugars along with some disaccharides, some oligosaccharides, and some polysaccharides.
The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. Examples of monosaccharide reducing sugars are galactose, glucose, fructose, etc.
A disaccharide is formed from two monosaccharides. A disaccharide can be classified as a reducing sugar or a non – reducing sugar based on presence of a hydroxyl group at the anomeric carbon. If the hydroxyl group is present at the anomeric carbon, then the disaccharide is reducing, else it is a non – reducing sugar.
In the given question, sucrose is a non-reducing sugar due to the absence of hydroxyl group at the anomeric carbon. It is a dimer of glucose and fructose. It is also known as cane sugar. The structure of sucrose is:

Hence, the correct answer is (A) cane sugar.
Note: Remember that a ketose sugar first tautomerizes into an aldose sugar and then it is reduced. It can’t be oxidised in ketose form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




