
An electric tester is used to check the conductivity of (a) pure water (b) vegetable oil (c) sugar solution as shown in the figure.
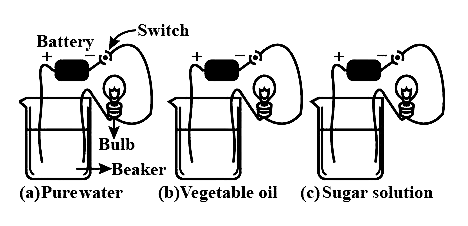
Choose the correct statement:
A. Bulb will glow in case (a) and case (b).
B. Bulb will glow in case (b) only.
C. Bulb will glow in case (b) and case (c).
D. Bulbs will glow in any of the given cases.

Answer
564.9k+ views
Hint: According to the object's behaviour whether it is able to conduct electricity through it or not, an electric tester will be able to justify whether the bulb will glow or not. Conductors are able to conduct electricity while on the other hand insulators are those which do not readily conduct electricity. A solution conducts if there are free ions in it.
Complete answer: Tap water has some salts and minerals dissolved in it, which help in the conduction of electricity, thus it is a good conductor of electricity. But pure water does not contain any salts, therefore, it is known as a poor conductor of electricity.
Vegetable oil contains some impurities and salts these help in the conduction of electricity.
We know that glucose solutions do not conduct electricity. Here, when we dissolve sugar in water to make a solution, it does not dissociate into ions. That is why it is considered a poor conductor.
Hence, clearly, option (B) is the correct answer.
Note: Electrical conductivity is the ability of a material to conduct electricity. If we read the explanation carefully, we will notice that here two terms are used, i.e., good conductors and poor conductors. But terms like bad conductors and insulators are not used. This is because some material mediums can conduct electricity but only under certain specific circumstances. This is the reason why we classify all the substances into good and poor conductors, and not as conductors and insulators. Hence, one needs to be careful while using such terms.
Complete answer: Tap water has some salts and minerals dissolved in it, which help in the conduction of electricity, thus it is a good conductor of electricity. But pure water does not contain any salts, therefore, it is known as a poor conductor of electricity.
Vegetable oil contains some impurities and salts these help in the conduction of electricity.
We know that glucose solutions do not conduct electricity. Here, when we dissolve sugar in water to make a solution, it does not dissociate into ions. That is why it is considered a poor conductor.
Hence, clearly, option (B) is the correct answer.
Note: Electrical conductivity is the ability of a material to conduct electricity. If we read the explanation carefully, we will notice that here two terms are used, i.e., good conductors and poor conductors. But terms like bad conductors and insulators are not used. This is because some material mediums can conduct electricity but only under certain specific circumstances. This is the reason why we classify all the substances into good and poor conductors, and not as conductors and insulators. Hence, one needs to be careful while using such terms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




