
An aqueous solution of A gives yellow precipitate with $K_2Cr_2O_4$. A may contain
A.$P{b^{2 + }}$ or $A{g^ + }$ or $B{a^{2 + }}$
B.$P{b^{2 + }}$or $B{a^{2 + }}$
C.$A{g^ + }$ or $B{a^{2 + }}$
D.$P{b^{2 + }}$ or $A{g^ + }$
Answer
567k+ views
Hint: The inorganic ions are categorized into different groups for their qualitative analysis. Inorganic salts consist of two parts known as radicals. The positively charged part (cation) is called a basic radical and the negatively charged part of the salt (anion) is called an acidic radical.
Complete step by step answer:
-Qualitative analysis involves the identification of cations and anions which are present in the salt or a mixture of salts. In this type of analysis we try to find out the nature of substance and the identity of the constituents.
-Inorganic qualitative analysis mainly involves the identification of cations and anions with the help of various reagents. The cations are mainly categorized into six different groups. There is systematic analysis.
-Potassium chromate $left( {{K_2}Cr{O_4}} right)$ is used to detect the presence of $P{b^{2 + }}$ and [B{a^{2 + }}]ions.
-In the first group lead is precipitated as lead chloride. On adding a potassium chromate solution, a yellow precipitate of lead chromate is obtained. This confirms the presence of lead ions.
$PbC{l_2}\, + \,{K_2}Cr{O_4} \to PbCr{O_4}\, + \,2KCl$
-The group five cations are precipitated as their carbonates which dissolve in acetic acid due to the formation of corresponding acetates.
-Potassium chromate solution gives a yellow precipitate of barium carbonate when the solution of fifth group precipitate in acetic acid is treated with it.
$BaC{O_3}\, + \,C{H_3}COOH\, \to {(C{H_3}COOH)_2}Ba\, + \,{H_2}O\, + \,C{O_2}$
${(C{H_3}COO)_2}Ba\, + \,{K_2}Cr{O_4}\, \to BaCr{O_4}\, + \,2C{H_3}COOK$
-So we can see that lead and barium ions are detected with the help of potassium chromate solution.
Hence the correct option is B.
Note:
-The structure of potassium chromate is
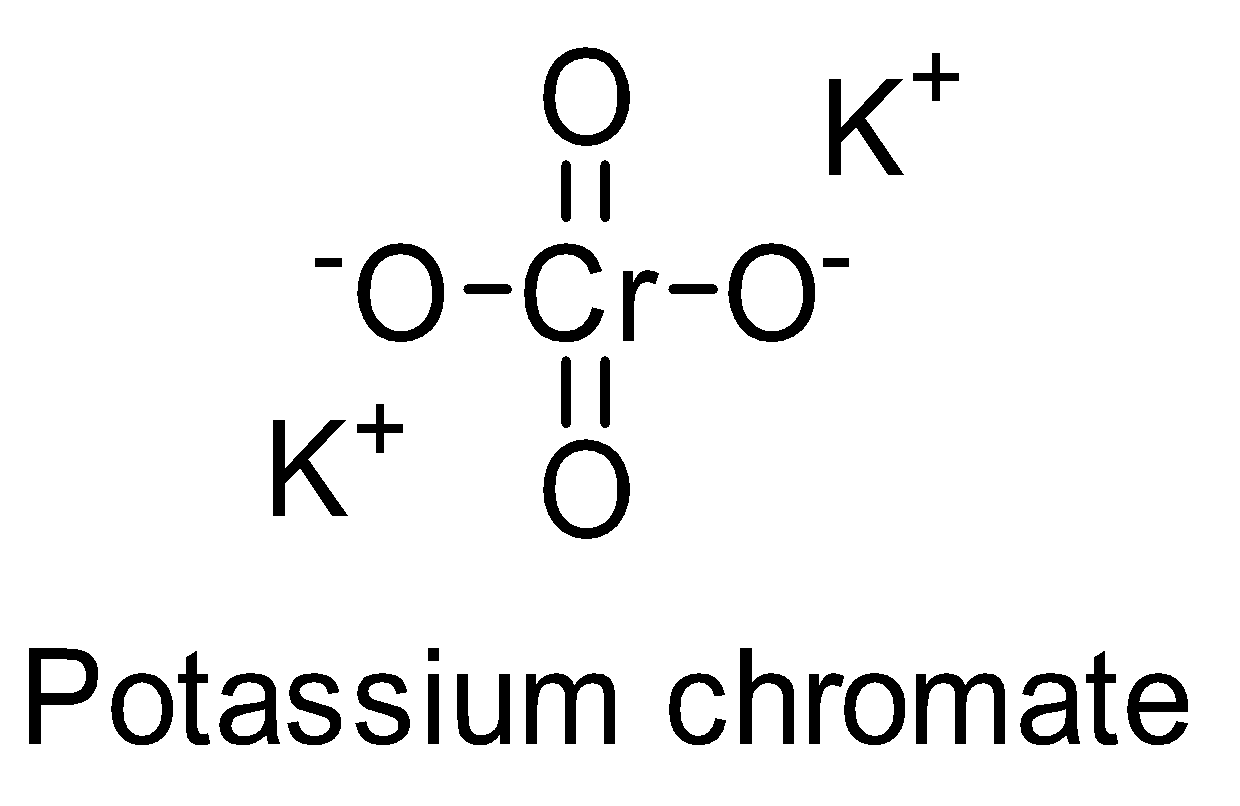
-Potassium chromate is a water-soluble compound. It appears as a yellow crystalline solid. Its mineral form is called tarapacaite.
Complete step by step answer:
-Qualitative analysis involves the identification of cations and anions which are present in the salt or a mixture of salts. In this type of analysis we try to find out the nature of substance and the identity of the constituents.
-Inorganic qualitative analysis mainly involves the identification of cations and anions with the help of various reagents. The cations are mainly categorized into six different groups. There is systematic analysis.
-Potassium chromate $left( {{K_2}Cr{O_4}} right)$ is used to detect the presence of $P{b^{2 + }}$ and [B{a^{2 + }}]ions.
-In the first group lead is precipitated as lead chloride. On adding a potassium chromate solution, a yellow precipitate of lead chromate is obtained. This confirms the presence of lead ions.
$PbC{l_2}\, + \,{K_2}Cr{O_4} \to PbCr{O_4}\, + \,2KCl$
-The group five cations are precipitated as their carbonates which dissolve in acetic acid due to the formation of corresponding acetates.
-Potassium chromate solution gives a yellow precipitate of barium carbonate when the solution of fifth group precipitate in acetic acid is treated with it.
$BaC{O_3}\, + \,C{H_3}COOH\, \to {(C{H_3}COOH)_2}Ba\, + \,{H_2}O\, + \,C{O_2}$
${(C{H_3}COO)_2}Ba\, + \,{K_2}Cr{O_4}\, \to BaCr{O_4}\, + \,2C{H_3}COOK$
-So we can see that lead and barium ions are detected with the help of potassium chromate solution.
Hence the correct option is B.
Note:
-The structure of potassium chromate is

-Potassium chromate is a water-soluble compound. It appears as a yellow crystalline solid. Its mineral form is called tarapacaite.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




