
An alkene “A” on reaction with ${{O}_{3}}$ and $Zn-{{H}_{2}}O$ gives propanone and ethanol in equimolar ratio. The addition of $HCl$ to alkene "A" gives "B" as the major product. The structure of the product "B" is:
(A)
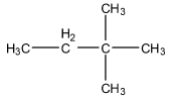
(B)
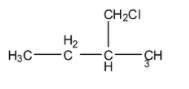
(C)
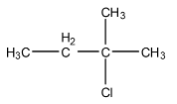
(D)
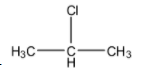




Answer
578.7k+ views
Hint: Alkene is a compound in which there is a double bond and when it is treated with ozone the double bond will break and will form two compounds having the carbonyl group. In a double, the chloride ion can attack either side, but the major product will have more stability.
Complete step by step solution:
The alkene is a compound in which there is a double bond and when it is treated with ozone the double bond will break and will form two compounds having the carbonyl group. And the question is given that the two products formed are propanone and ethanol.
The structure of propanone is given below:
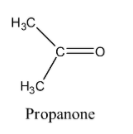
The structure of ethanol is given below:
$C{{H}_{3}}-CHO$
So, the alkene will be 2-Methyl but-2-ene and its structure is given below:
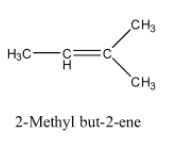
The reaction of 2-Methyl but-2-ene with ${{O}_{3}}$ and $Zn-{{H}_{2}}O$ is given below:
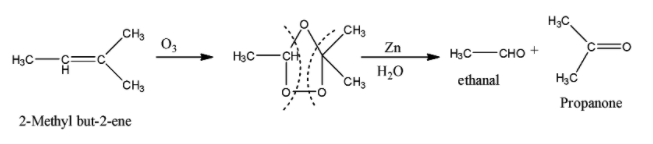
So when 2-Methyl but-2-ene is reacted with $HCl$, the addition of chloride ion takes place in such a manner that the major product is the one in which the chlorine atom is attached to the tertiary carbon atom. So the reaction is given below:
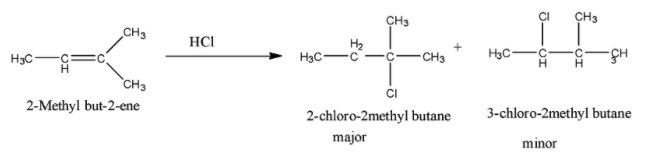
Therefore, the major product is 2-chloro-2methyl methyl butane.
Hence, the correct answer is an option (C).
Note: It must be noted that the addition of $HCl$to the alkene takes place through Markovnikov’s rule i.e., the negative part of the reagent will get attached to the carbon atom having a lesser number of hydrogen atoms.
Complete step by step solution:
The alkene is a compound in which there is a double bond and when it is treated with ozone the double bond will break and will form two compounds having the carbonyl group. And the question is given that the two products formed are propanone and ethanol.
The structure of propanone is given below:

The structure of ethanol is given below:
$C{{H}_{3}}-CHO$
So, the alkene will be 2-Methyl but-2-ene and its structure is given below:

The reaction of 2-Methyl but-2-ene with ${{O}_{3}}$ and $Zn-{{H}_{2}}O$ is given below:

So when 2-Methyl but-2-ene is reacted with $HCl$, the addition of chloride ion takes place in such a manner that the major product is the one in which the chlorine atom is attached to the tertiary carbon atom. So the reaction is given below:

Therefore, the major product is 2-chloro-2methyl methyl butane.
Hence, the correct answer is an option (C).
Note: It must be noted that the addition of $HCl$to the alkene takes place through Markovnikov’s rule i.e., the negative part of the reagent will get attached to the carbon atom having a lesser number of hydrogen atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




