
An alcoholic solution of dimethylglyoxime is added to an aqueous solution of nickel (II) chloride. Slow addition of ammonium hydroxide leads to the precipitation of a rosy red coloured metal complex. Then find out the number of hydrogen bonds present in the structure of the complex.
(A) 2
(B) 3
(C) 3
(D) None
Answer
541.8k+ views
Hint: The red coloured complex is the product in the form of precipitate which is produced when an alcoholic solution of dimethylglyoxime is added to an aqueous solution of nickel (II) chloride.
Also, it’s a hint that the valency of nickel will determine the complex formation and the number of hydrogen bonds formed.
Complete step-by-step answer: Let us discuss the reactants involved to form a product whose hydrogen bonds needs to be counted;
Dimethylglyoxime is the whitish crystalline solid with molecular formula of ${{C}_{4}}{{H}_{8}}{{N}_{2}}{{O}_{2}}$ (also known as 2, 3 – butadiene dioxide).
When $dmg{{H}_{2}}$ reacts with metals salts, a complex is formed i.e. metal-dmg is formed. So, in the case of nickel (II) chloride; the following displayed complex is formed.
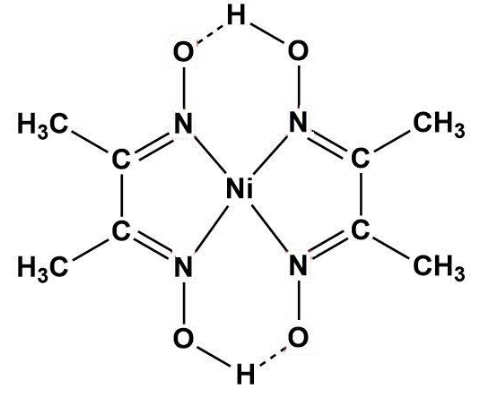
Here, we can see that when $N{{i}^{2+}}$ reacts with $dmg{{H}_{2}}$, a Ni-dmg complex is formed having two hydrogen bonds (also as the valency of nickel is 2 in ionic form).
Therefore option (2) is correct.
Note:Dimethylglyoxime is actually used for the analysis and identification of nickel and palladium.
Specifically, for the nickel atom, the white coloured powder changes to a rosy red colour complex; hence, the presence of nickel is determined.
Also, it’s a hint that the valency of nickel will determine the complex formation and the number of hydrogen bonds formed.
Complete step-by-step answer: Let us discuss the reactants involved to form a product whose hydrogen bonds needs to be counted;
Dimethylglyoxime is the whitish crystalline solid with molecular formula of ${{C}_{4}}{{H}_{8}}{{N}_{2}}{{O}_{2}}$ (also known as 2, 3 – butadiene dioxide).
When $dmg{{H}_{2}}$ reacts with metals salts, a complex is formed i.e. metal-dmg is formed. So, in the case of nickel (II) chloride; the following displayed complex is formed.

Here, we can see that when $N{{i}^{2+}}$ reacts with $dmg{{H}_{2}}$, a Ni-dmg complex is formed having two hydrogen bonds (also as the valency of nickel is 2 in ionic form).
Therefore option (2) is correct.
Note:Dimethylglyoxime is actually used for the analysis and identification of nickel and palladium.
Specifically, for the nickel atom, the white coloured powder changes to a rosy red colour complex; hence, the presence of nickel is determined.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




