
An alcohol X when treated with hot conc. ${H_2}S{O_4}$ gave an alkene Y with formula ${C_4}{H_8}$ . This alkene on ozonolysis gives a single product with molecular formula ${C_2}{H_4}O$ . The alcohol is
A.Butan $ - 1 - $ ol
B.Butan $ - 2 - $ ol
C.$2 - $ methylpropan $ - 1 - $ ol
D.$2,2 - $ dimethylbutan $ - 1 - $ ol
Answer
570k+ views
Hint:
Ozonolysis reaction is used to identify the position of double bonds in alkenes or other unsaturated compounds. In an ozonolysis reaction, the substrate reacts with an ozone molecule to give ozonide which when further treated with zinc gives the final product. The final product can be a ketone or aldehyde.
Complete step by step answer:
Definition: Ozonolysis is a reaction in which an ozone molecule is added to the unsaturated bond in a molecule to form ozonide and then this ozonide is cleaved or broken into smaller molecules. This reaction is usually carried out with alkenes.
In ozonolysis the carbon-carbon bond in alkenes or alkynes is replaced by a carbonyl group.
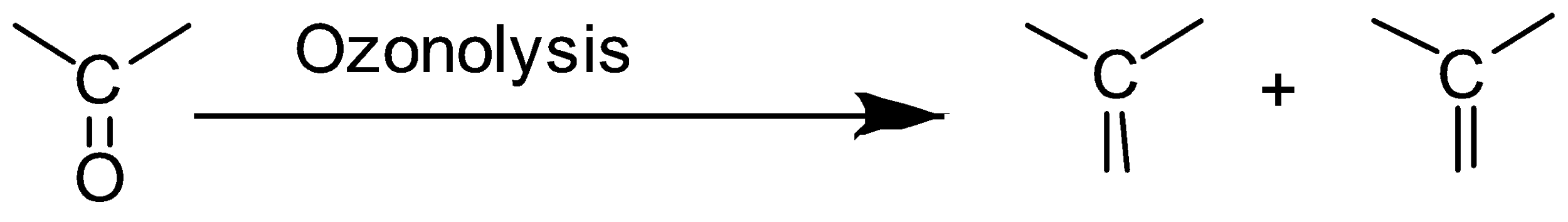
According to the given question,
$X(ROH) + {H_2}S{O_4} \to Y({C_4}{H_8})\xrightarrow{{ozonolysis}}{C_2}{H_4}O$
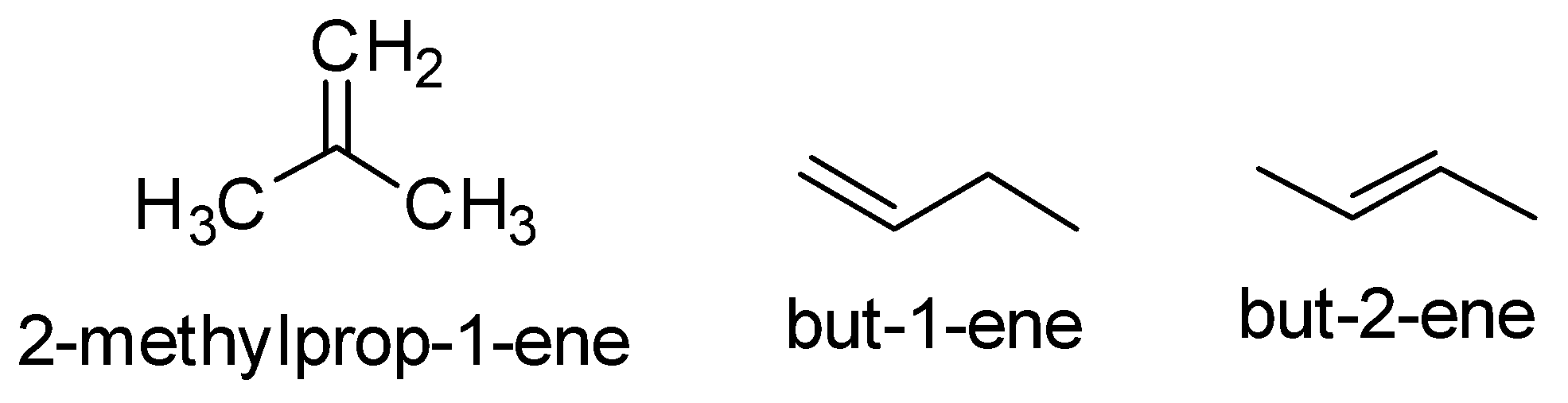
Now the alkene which is formed in the given reaction is ${C_4}{H_8}$ . So the possible alkene are
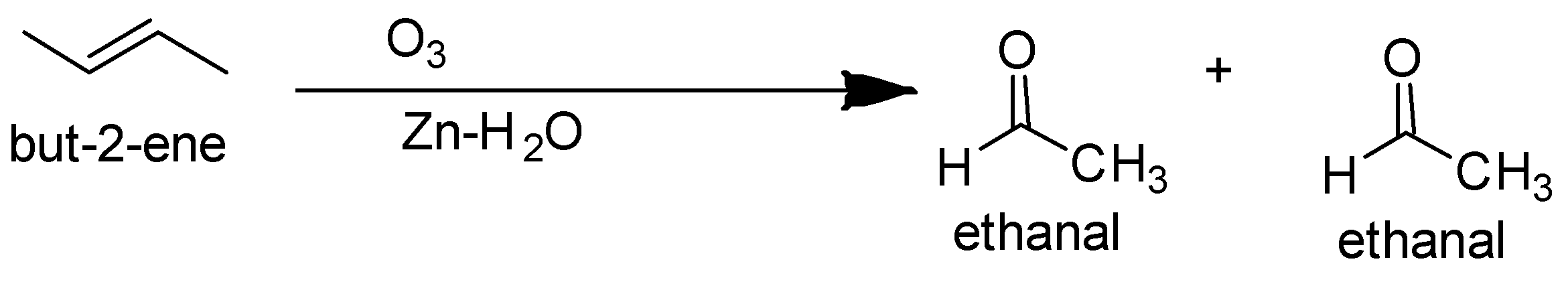
So the ozonolysis of only but $ - 2 - $ ene gives a single product. The reaction is as follows-
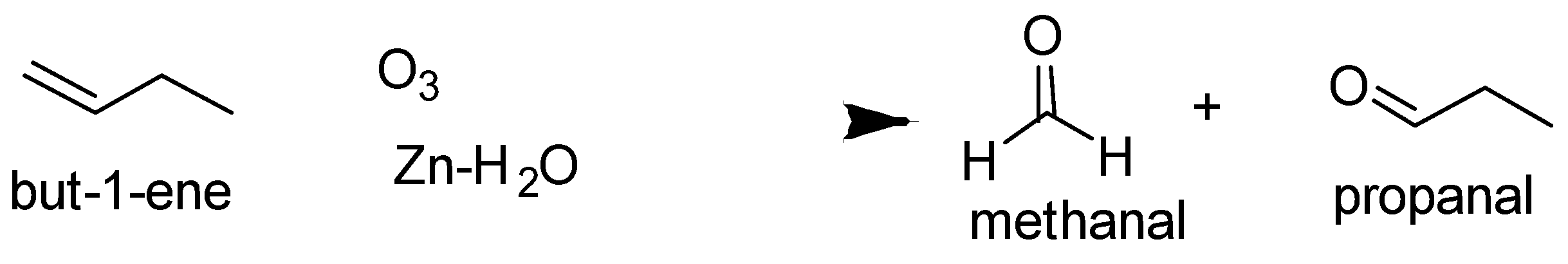
The ozonolysis of but $ - 1 - $ ene and $2 - $ methylprop $ - 1 - $ ene are as follows-
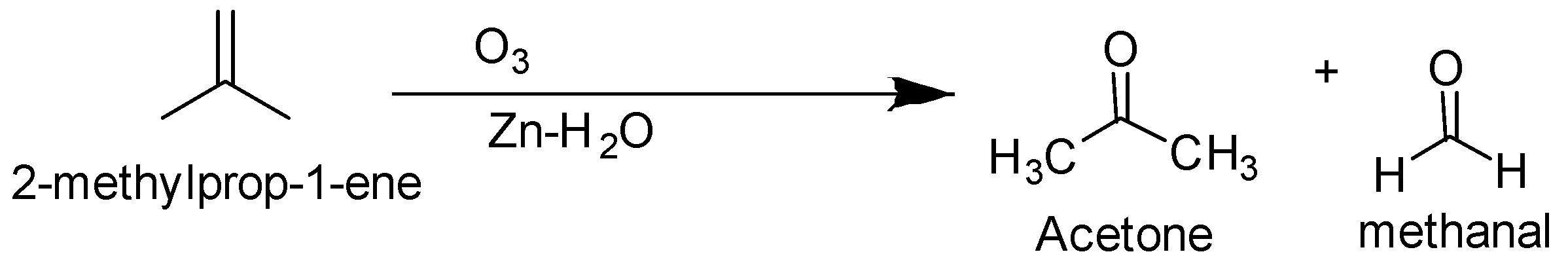
So the alkene is but $ - 2 - $ ene .
When alcohol undergoes acidic hydrolysis dehydration of alcohol takes place and alkene is formed.
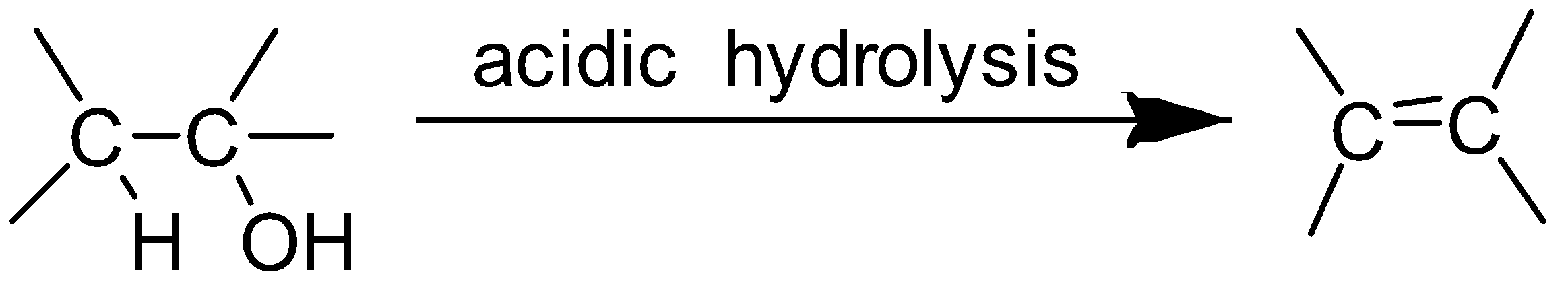
The alcohol which on acidic hydrolysis gives but $ - 2 - $ ene is butan $ - 2 - $ ol.
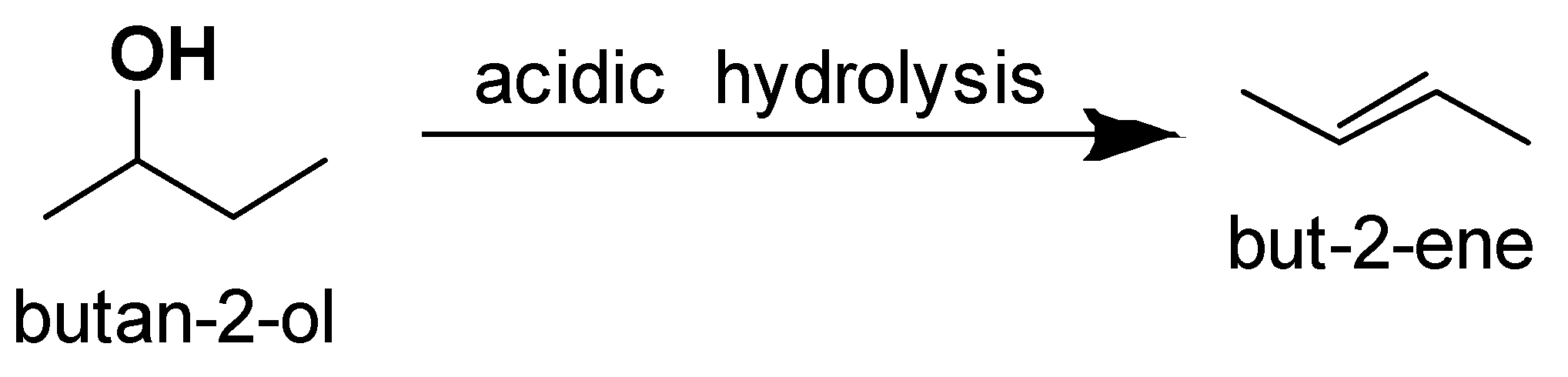
So the correct option is B.
Note:The reagents required for ozonolysis are ${O_{{3_{}}}}\,and\,Zn - {H_2}O$ .
-There is a trick for identifying the ozonolysis reaction product. Cleave the carbon-carbon bond and convert the end carbon atoms of the double bond into carbonyl groups. Zinc prevents the molecule from further forming a bond with oxygen molecules. It prevents further oxidation.
-Dehydration is the process of removal of water molecules.
Ozonolysis reaction is used to identify the position of double bonds in alkenes or other unsaturated compounds. In an ozonolysis reaction, the substrate reacts with an ozone molecule to give ozonide which when further treated with zinc gives the final product. The final product can be a ketone or aldehyde.
Complete step by step answer:
Definition: Ozonolysis is a reaction in which an ozone molecule is added to the unsaturated bond in a molecule to form ozonide and then this ozonide is cleaved or broken into smaller molecules. This reaction is usually carried out with alkenes.
In ozonolysis the carbon-carbon bond in alkenes or alkynes is replaced by a carbonyl group.

According to the given question,
$X(ROH) + {H_2}S{O_4} \to Y({C_4}{H_8})\xrightarrow{{ozonolysis}}{C_2}{H_4}O$
Now the alkene which is formed in the given reaction is ${C_4}{H_8}$ . So the possible alkene are

So the ozonolysis of only but $ - 2 - $ ene gives a single product. The reaction is as follows-

The ozonolysis of but $ - 1 - $ ene and $2 - $ methylprop $ - 1 - $ ene are as follows-


So the alkene is but $ - 2 - $ ene .
When alcohol undergoes acidic hydrolysis dehydration of alcohol takes place and alkene is formed.
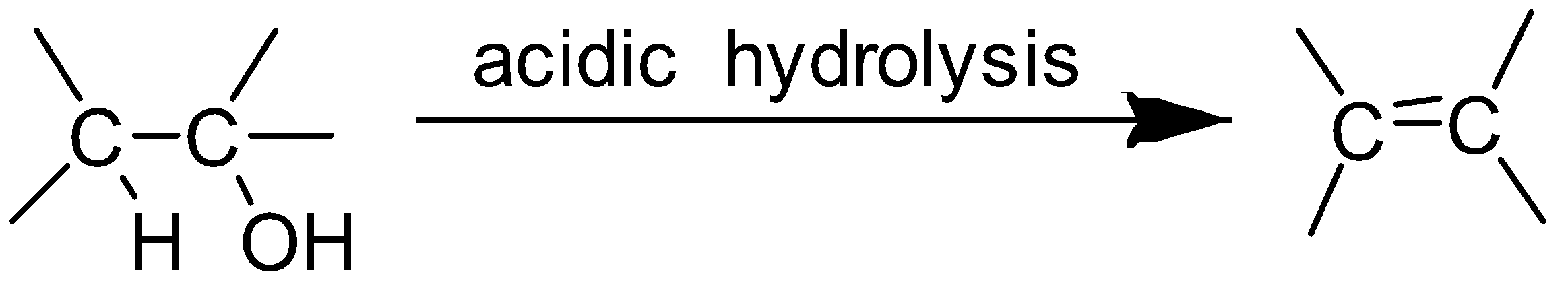
The alcohol which on acidic hydrolysis gives but $ - 2 - $ ene is butan $ - 2 - $ ol.
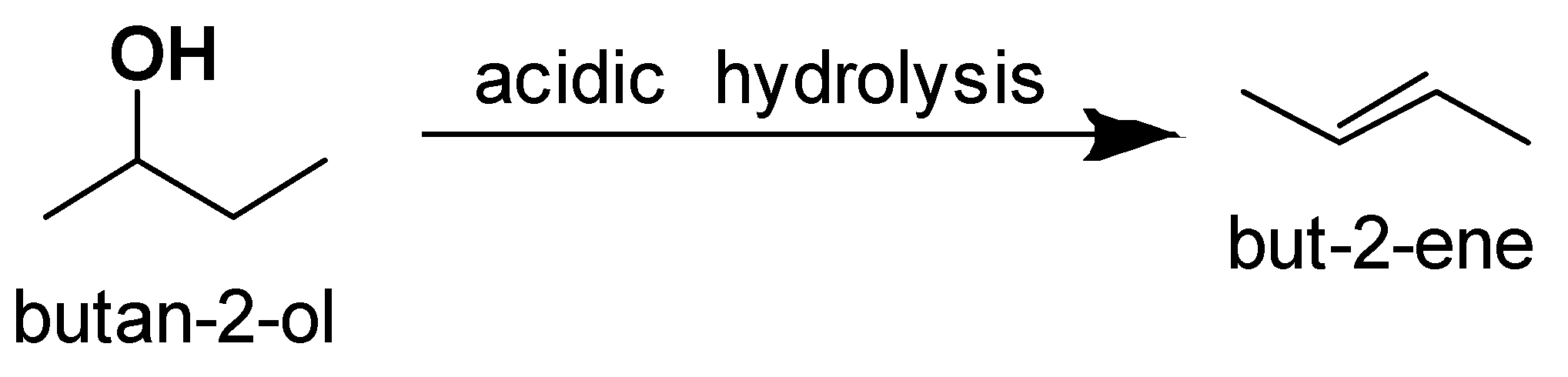
So the correct option is B.
Note:The reagents required for ozonolysis are ${O_{{3_{}}}}\,and\,Zn - {H_2}O$ .
-There is a trick for identifying the ozonolysis reaction product. Cleave the carbon-carbon bond and convert the end carbon atoms of the double bond into carbonyl groups. Zinc prevents the molecule from further forming a bond with oxygen molecules. It prevents further oxidation.
-Dehydration is the process of removal of water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




