
What amount of bromine will be required to convert $2g$ of phenol into $2,4,6 - $ tribromophenol?
A. $4.0$
B. $6.0$
C. $10.2$
D. $20.4$
Answer
578.4k+ views
Hint: The stoichiometry of the reaction is the deciding factor of about how much weight of any reactant do you require yielding the product. The reactants react with each other in a simple integral ratio and this helps in defining the physical analysis of the reacting materials.
Complete step by step answer:
Phenol is an aromatic organic compound with the molecular formula \[{C_6}{H_5}OH\] . It is a white crystalline volatile solid. The molecule consists of a phenyl group bonded to a hydroxyl group. Being mildly acidic, it requires careful handling because it can cause chemical burns on the skin and can even cause irradiation of eyes.
When phenol reacts with excess of bromine, it produces $2,4,6 - $ tribromophenol. The reaction is as follows:
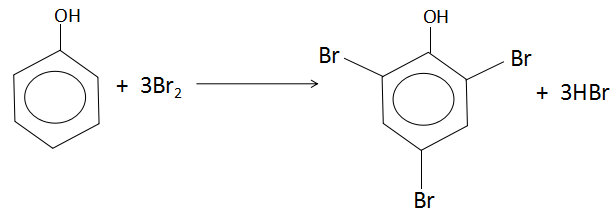
As per the reaction, one mole of phenol reacts with three moles of bromine. One mole of any substance is equal to the molar mass of that substance. Let us calculate the molar masses of the reactants.
Phenol = ${C_6}{H_5}OH = (6 \times 12) + (6 \times 1) + (1 \times 16) = 94g$
Bromine = $B{r_2} = 160g$
Hence, we can clearly see from the reaction that $1mole$ of phenol reacts with $3moles$ of bromine. This means:
$(1 \times 94)g$ of phenol reacts with = $(3 \times 160)g$ of bromine
$1g$ of phenol will react with = $\dfrac{{3 \times 160}}{{94}}g$ of bromine
Hence, $2g$ of phenol will react with = $2 \times \dfrac{{3 \times 160}}{{94}} = 10.2g$ of bromine
Thus, the correct option is C. $10.2$ .
Note:
The water that we see in hydrated salt is called water of crystallisation. This even imparts
colour to salts. We should not overheat the salt, so much that the salt itself will start dissociation.
But we need only loss of water, so heating has to be done under proper care.
Complete step by step answer:
Phenol is an aromatic organic compound with the molecular formula \[{C_6}{H_5}OH\] . It is a white crystalline volatile solid. The molecule consists of a phenyl group bonded to a hydroxyl group. Being mildly acidic, it requires careful handling because it can cause chemical burns on the skin and can even cause irradiation of eyes.
When phenol reacts with excess of bromine, it produces $2,4,6 - $ tribromophenol. The reaction is as follows:

As per the reaction, one mole of phenol reacts with three moles of bromine. One mole of any substance is equal to the molar mass of that substance. Let us calculate the molar masses of the reactants.
Phenol = ${C_6}{H_5}OH = (6 \times 12) + (6 \times 1) + (1 \times 16) = 94g$
Bromine = $B{r_2} = 160g$
Hence, we can clearly see from the reaction that $1mole$ of phenol reacts with $3moles$ of bromine. This means:
$(1 \times 94)g$ of phenol reacts with = $(3 \times 160)g$ of bromine
$1g$ of phenol will react with = $\dfrac{{3 \times 160}}{{94}}g$ of bromine
Hence, $2g$ of phenol will react with = $2 \times \dfrac{{3 \times 160}}{{94}} = 10.2g$ of bromine
Thus, the correct option is C. $10.2$ .
Note:
The water that we see in hydrated salt is called water of crystallisation. This even imparts
colour to salts. We should not overheat the salt, so much that the salt itself will start dissociation.
But we need only loss of water, so heating has to be done under proper care.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




