
Amongst the following the lowest degree of paramagnetism per mol of the compound at $298{\text{ K}}$ will be shown by:
A) ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$
B) ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$
C) ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$
D) ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$
Answer
578.4k+ views
Hint: We know that the complexes having unpaired electrons are paramagnetic in nature and those having no unpaired electrons are diamagnetic in nature. The crystal field theory helps to describe if the compound is paramagnetic or diamagnetic.
Complete step by step answer:
The ligand bonds to the central metal atom and donates a pair of electrons to the central metal atom and thus, form a coordination complex.
We are given four complexes, ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$, ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$, ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ and ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$. In the given complexes, the ligand is the water molecule.
Water is a weak field ligand. Thus, it will not cause the pairing of the electrons of the metal atom and high spin complexes are formed.
The oxidation state of ${\text{Fe}}$ in ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{F}}{{\text{e}}^{2 + }}$ is $3{d^6}4{s^0}$. Thus,
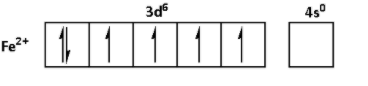
The oxidation state of ${\text{Ni}}$ in ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{N}}{{\text{i}}^{2 + }}$ is $3{d^8}4{s^0}$. Thus,
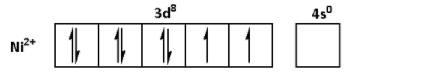
The oxidation state of ${\text{Mn}}$ in ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{M}}{{\text{n}}^{2 + }}$ is $3{d^5}4{s^0}$. Thus,
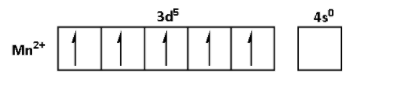
The oxidation state of ${\text{Cu}}$ in ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{C}}{{\text{u}}^{2 + }}$is $3{d^9}4{s^0}$. Thus,
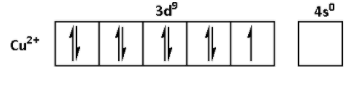
From the electronic configurations, we can see that the ${\text{M}}{{\text{n}}^{2 + }}$ has five unpaired electrons. Thus, ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ shows highest degree of paramagnetism. ${\text{F}}{{\text{e}}^{2 + }}$ has four unpaired electrons. Thus, the degree of paramagnetism of ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is lower than that of ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$. ${\text{N}}{{\text{i}}^{2 + }}$ has two unpaired electrons. Thus, the degree of paramagnetism of ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is lower than that of ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$. ${\text{C}}{{\text{u}}^{2 + }}$ has one unpaired electron. Thus, ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$ shows lowest degree of paramagnetism.
Thus, the correct option is (D) ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$.
Note: Strong field ligands form low spin complexes. The examples of strong field ligands are ${\text{C}}{{\text{O}}^ - }$, ${\text{C}}{{\text{N}}^ - }$. Weak field ligands form high spin complexes. The examples of weak field ligands are ${{\text{F}}^ - }$, ${\text{C}}{{\text{l}}^ - }$, ${{\text{H}}_{\text{2}}}{\text{O}}$.
Complete step by step answer:
The ligand bonds to the central metal atom and donates a pair of electrons to the central metal atom and thus, form a coordination complex.
We are given four complexes, ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$, ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$, ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ and ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$. In the given complexes, the ligand is the water molecule.
Water is a weak field ligand. Thus, it will not cause the pairing of the electrons of the metal atom and high spin complexes are formed.
The oxidation state of ${\text{Fe}}$ in ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{F}}{{\text{e}}^{2 + }}$ is $3{d^6}4{s^0}$. Thus,

The oxidation state of ${\text{Ni}}$ in ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{N}}{{\text{i}}^{2 + }}$ is $3{d^8}4{s^0}$. Thus,

The oxidation state of ${\text{Mn}}$ in ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{M}}{{\text{n}}^{2 + }}$ is $3{d^5}4{s^0}$. Thus,

The oxidation state of ${\text{Cu}}$ in ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$ is +2. Thus, the outer shell electronic configuration of ${\text{C}}{{\text{u}}^{2 + }}$is $3{d^9}4{s^0}$. Thus,

From the electronic configurations, we can see that the ${\text{M}}{{\text{n}}^{2 + }}$ has five unpaired electrons. Thus, ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$ shows highest degree of paramagnetism. ${\text{F}}{{\text{e}}^{2 + }}$ has four unpaired electrons. Thus, the degree of paramagnetism of ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is lower than that of ${\text{MnS}}{{\text{O}}_{\text{4}}}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}$. ${\text{N}}{{\text{i}}^{2 + }}$ has two unpaired electrons. Thus, the degree of paramagnetism of ${\text{NiS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$ is lower than that of ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}$. ${\text{C}}{{\text{u}}^{2 + }}$ has one unpaired electron. Thus, ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$ shows lowest degree of paramagnetism.
Thus, the correct option is (D) ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$.
Note: Strong field ligands form low spin complexes. The examples of strong field ligands are ${\text{C}}{{\text{O}}^ - }$, ${\text{C}}{{\text{N}}^ - }$. Weak field ligands form high spin complexes. The examples of weak field ligands are ${{\text{F}}^ - }$, ${\text{C}}{{\text{l}}^ - }$, ${{\text{H}}_{\text{2}}}{\text{O}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




