
Among the following, which is antiaromatic
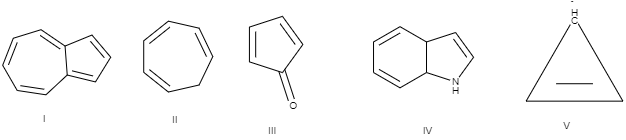
A.\[I\text{ }and\text{ }IV\]
B.\[III\text{ }and\text{ }IV\]
C.\[II\text{ }and\text{ }V\]
D.\[I\text{ }and\text{ }III\]

Answer
579k+ views
Hint: Antiaromatic molecules or ions have a characteristic of a cyclic molecule with a pi electron system that are of higher energy due to the presence of delocalised electrons in it.
Unlike the aromatic compounds, which are highly stable and follow Hückel's rule, antiaromatic compounds are highly reactive and unstable and do not follow Huckel’s rule .
Complete step by step answer:
Aromatic compounds are a type of chemical compounds which consist of conjugated planar ring systems along with delocalized pi-electron clouds in place of individual, having alternating double and single bonds.
They are also known as aromatics or arenes. The most common examples are benzene and toluene. For a compound to be Aromatics, it requires satisfying the Huckel’s rule. Microorganisms and Plants have an exclusive route to aromatic compounds having benzene rings. Therefore a huge majority of aromatic compounds in nature are produced by microorganisms and plants, and animals are dependent upon plants for many aromatic compounds either directly or indirectly.
On the other hand, anti-aromatic compounds can be defined as a cyclic compound which doesn’t necessarily have a continuous form of overlapping ring of p orbitals must not be considered as aromatic or even anti aromatic and hence these are known as nonaromatic or aliphatic compounds.
Non-aromatic compounds' electronic energy is like that of its open-chain counterpart. Crude oil comprises mainly alkanes and large releases into the sea due to the wreckage of oil tankers results in the death of many seabirds and other marine organisms because of their physical effects of oiling or smothering with these compounds.
Anti-aromatic compounds are those which are cyclic molecules and have a planar structure in which all the bonded atoms lie in the same plane.
The basic difference between aromatic and antiaromatic compounds is that aromatic compounds include the benzene ring type structures having alternate double bonds but anti aromatic compounds doesn’t.
If we look at the options, B satisfies all the criteria necessary for a compound to be antiaromatic.
Hence the correct answer is option B.
Note:
Non aromatic compounds do not show any resonance in the compound.
Some examples of non aromatic compounds are alkanes, alkenes, alkynes. And that of anti aromatic compounds are cyclobutadiene, cyclohexane etc.
Unlike the aromatic compounds, which are highly stable and follow Hückel's rule, antiaromatic compounds are highly reactive and unstable and do not follow Huckel’s rule .
Complete step by step answer:
Aromatic compounds are a type of chemical compounds which consist of conjugated planar ring systems along with delocalized pi-electron clouds in place of individual, having alternating double and single bonds.
They are also known as aromatics or arenes. The most common examples are benzene and toluene. For a compound to be Aromatics, it requires satisfying the Huckel’s rule. Microorganisms and Plants have an exclusive route to aromatic compounds having benzene rings. Therefore a huge majority of aromatic compounds in nature are produced by microorganisms and plants, and animals are dependent upon plants for many aromatic compounds either directly or indirectly.
On the other hand, anti-aromatic compounds can be defined as a cyclic compound which doesn’t necessarily have a continuous form of overlapping ring of p orbitals must not be considered as aromatic or even anti aromatic and hence these are known as nonaromatic or aliphatic compounds.
Non-aromatic compounds' electronic energy is like that of its open-chain counterpart. Crude oil comprises mainly alkanes and large releases into the sea due to the wreckage of oil tankers results in the death of many seabirds and other marine organisms because of their physical effects of oiling or smothering with these compounds.
Anti-aromatic compounds are those which are cyclic molecules and have a planar structure in which all the bonded atoms lie in the same plane.
The basic difference between aromatic and antiaromatic compounds is that aromatic compounds include the benzene ring type structures having alternate double bonds but anti aromatic compounds doesn’t.
If we look at the options, B satisfies all the criteria necessary for a compound to be antiaromatic.
Hence the correct answer is option B.
Note:
Non aromatic compounds do not show any resonance in the compound.
Some examples of non aromatic compounds are alkanes, alkenes, alkynes. And that of anti aromatic compounds are cyclobutadiene, cyclohexane etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




