
Among the following, the reaction that proceeds through an electrophilic substitution is:

Answer
522.6k+ views
Hint: In a chemical reaction, the functional group attached to a compound is replaced by an electrophile, and then the reactions are known as electrophilic substitution reactions. The functional group which is replaced by a hydrogen atom is substituted in a chemical compound. Organic compounds involve two types of primary electrophilic substitution reactions which are electrophilic aromatic substitution and aliphatic substitution reactions.
Complete step by step answer:
Let us among given reactions, which one of the reactions belongs to electrophilic substitution reaction,
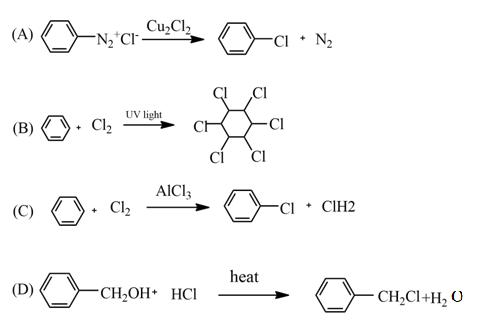
This reaction is a type of elimination reaction, which is classified as the two-step mechanism is known as E1 reaction and the one-step mechanism is known as E2 reaction.
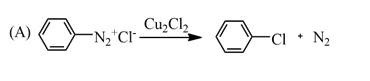
This reaction is a type of substitution reaction which belongs to radical aromatic substitution reactions. In this chlorine radical forms with a homolytic fission of chlorine molecule under UV light and this radical initiates the reaction and hence the reaction becomes a radical aromatic substitution reaction.
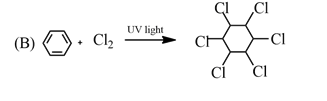
This reaction is named as Friedel-crafts reaction which is a type of electrophilic substitution reaction.
The mechanism of this reaction as follows:
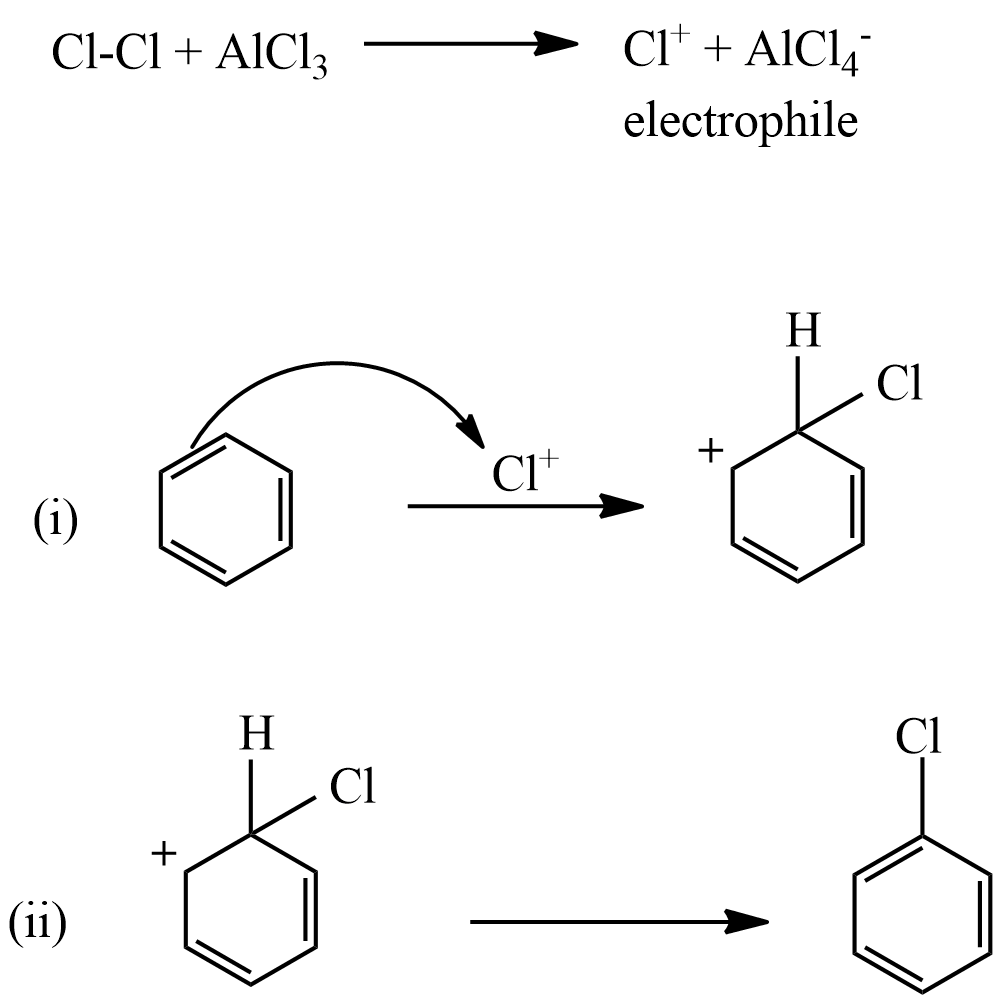
Hence, the reaction that proceeds through an electrophilic substitution.
The correct answer is option “C” .
Note: An atom that is attached to an aromatic ring is replaced with an electrophile in a reaction is an electrophilic aromatic substitution reaction. Aromatic nitration, aromatic sulfonation, and Friedel-Crafts reactions are examples of this type of reaction. The aromaticity of aromatic compounds plays an important role in electrophilic aromatic substitution reactions.
Complete step by step answer:
Let us among given reactions, which one of the reactions belongs to electrophilic substitution reaction,

This reaction is a type of elimination reaction, which is classified as the two-step mechanism is known as E1 reaction and the one-step mechanism is known as E2 reaction.

This reaction is a type of substitution reaction which belongs to radical aromatic substitution reactions. In this chlorine radical forms with a homolytic fission of chlorine molecule under UV light and this radical initiates the reaction and hence the reaction becomes a radical aromatic substitution reaction.

This reaction is named as Friedel-crafts reaction which is a type of electrophilic substitution reaction.
The mechanism of this reaction as follows:

Hence, the reaction that proceeds through an electrophilic substitution.
The correct answer is option “C” .
Note: An atom that is attached to an aromatic ring is replaced with an electrophile in a reaction is an electrophilic aromatic substitution reaction. Aromatic nitration, aromatic sulfonation, and Friedel-Crafts reactions are examples of this type of reaction. The aromaticity of aromatic compounds plays an important role in electrophilic aromatic substitution reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




