
Among the following, the alkene on ozonolysis gives rise to only one aldehyde as the product, is:
A. 1-butyne
B. Prop-1-ene
C. 2-butene
D. 2-methyl-prop-1-ene
Answer
527k+ views
Hint: “Ozonolysis is an organic reaction where the unsaturated bonds (double or triple bonds) of alkenes, alkynes, or azo compounds are cleaved by ozone”. On ozonolysis alkenes and alkynes form organic compounds in which the carbon–carbon bond (double or triple) has been replaced by a carbonyl group.
Complete step by step answer:
We can test the presence of double or triple bonds in the organic molecules by using ozonolysis.
Generally alkenes give aldehyde or ketone as the products on ozonolysis and alkynes gives carboxylic acids as a product on ozonolysis.
Coming to the given options, Option A, 1-butyne.
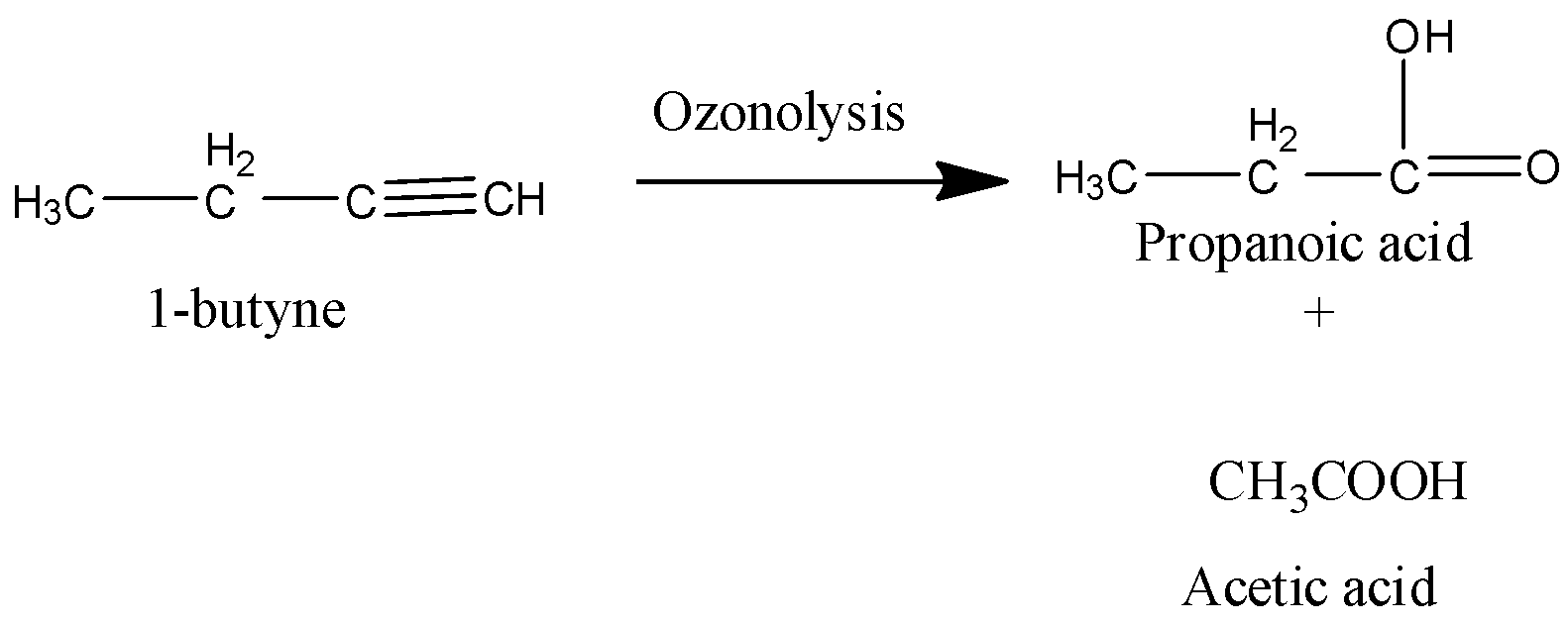
It is an alkyne which gives carboxylic acid as a product on hydrolysis.
Coming to option B, prop-1-ene.
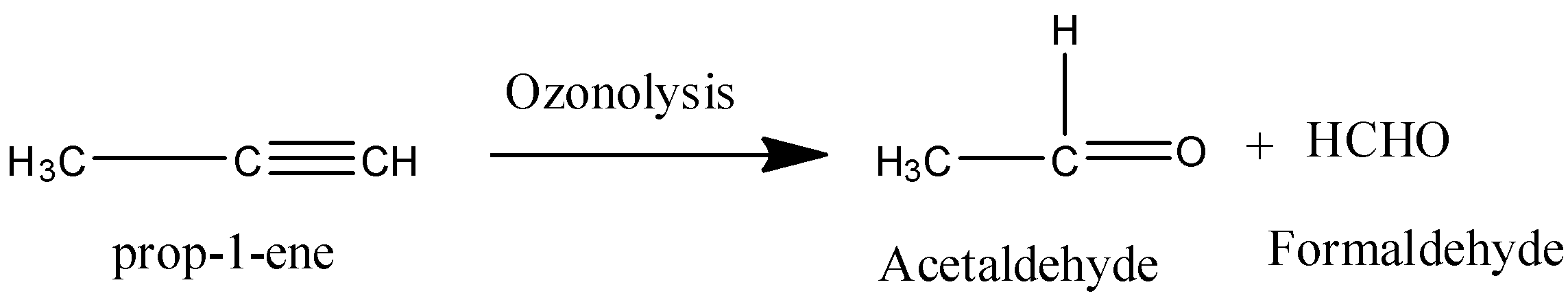
On ozonolysis prop-1-ene gives acetaldehyde and formaldehyde.
Coming to option C, 2-butene.
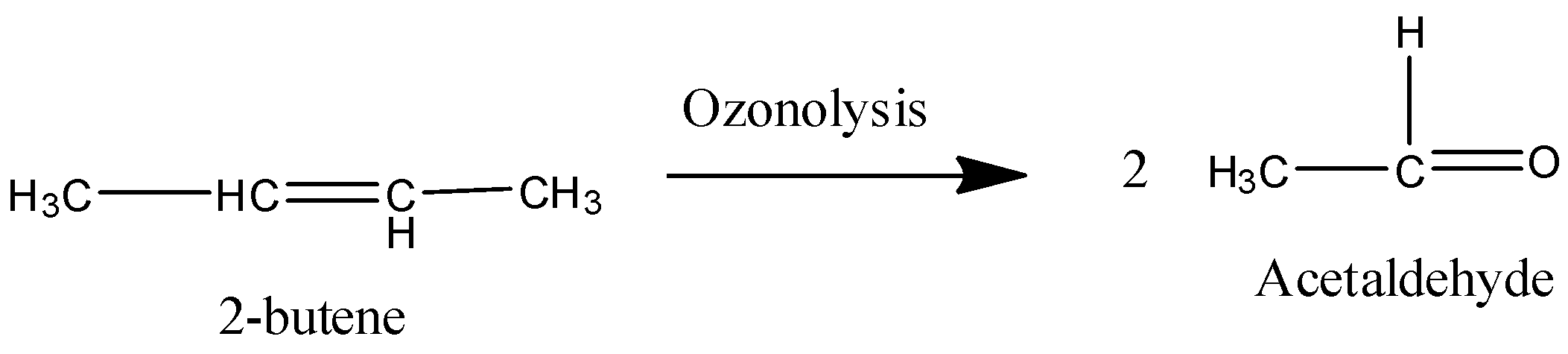
On ozonolysis 2-butene gives two moles of acetaldehyde.
Coming to option D, 2-methyl-prop-1-ene.
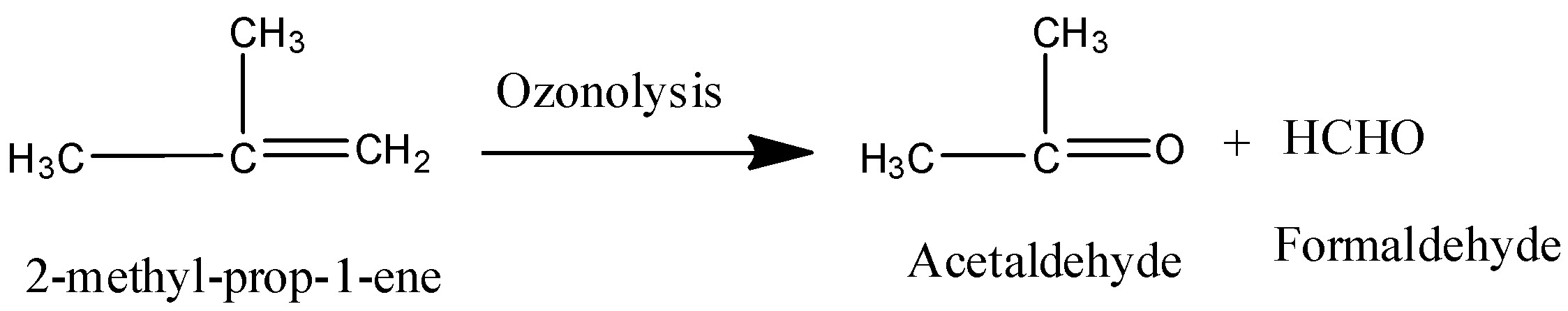
On ozonolysis 2-methyl-prop-1-ene gives acetaldehyde and formaldehyde.
So, the correct option is C, 2-butene gives two moles of only one aldehyde (acetaldehyde) on ozonolysis.
Note: Don’t be confused with alkenes and alkynes.
Alkene: The chemical which contains double bond in its structure is called alkene.
Alkyne: The chemical which contains triple bond in its structure is called alkyne.
We can differentiate alkenes and alkynes very easily by using ozonolysis. On ozonolysis alkene gives aldehyde or ketone as the products. But alkynes give carboxylic acids on ozonolysis.
Complete step by step answer:
We can test the presence of double or triple bonds in the organic molecules by using ozonolysis.
Generally alkenes give aldehyde or ketone as the products on ozonolysis and alkynes gives carboxylic acids as a product on ozonolysis.
Coming to the given options, Option A, 1-butyne.

It is an alkyne which gives carboxylic acid as a product on hydrolysis.
Coming to option B, prop-1-ene.

On ozonolysis prop-1-ene gives acetaldehyde and formaldehyde.
Coming to option C, 2-butene.
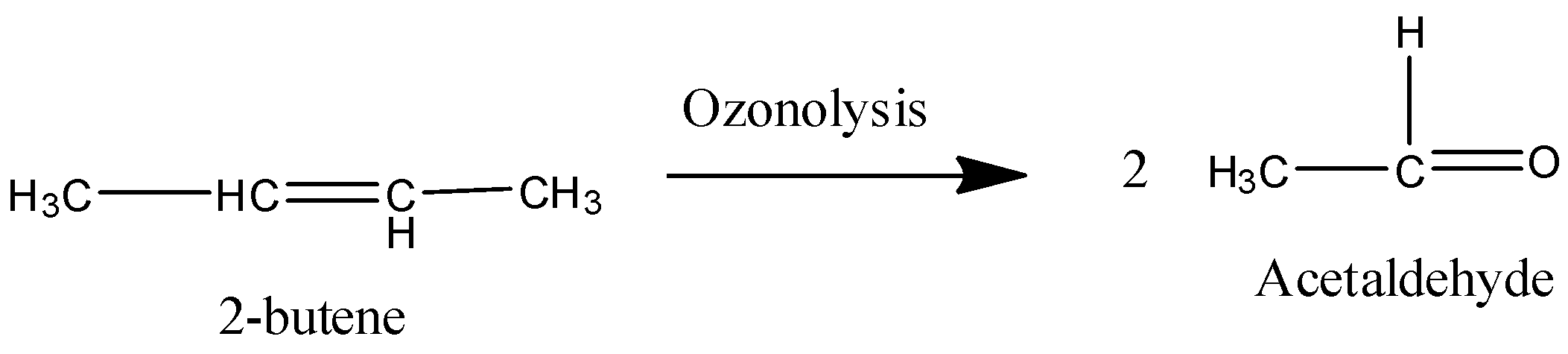
On ozonolysis 2-butene gives two moles of acetaldehyde.
Coming to option D, 2-methyl-prop-1-ene.

On ozonolysis 2-methyl-prop-1-ene gives acetaldehyde and formaldehyde.
So, the correct option is C, 2-butene gives two moles of only one aldehyde (acetaldehyde) on ozonolysis.
Note: Don’t be confused with alkenes and alkynes.
Alkene: The chemical which contains double bond in its structure is called alkene.
Alkyne: The chemical which contains triple bond in its structure is called alkyne.
We can differentiate alkenes and alkynes very easily by using ozonolysis. On ozonolysis alkene gives aldehyde or ketone as the products. But alkynes give carboxylic acids on ozonolysis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




