
Among the following compounds, the most basic compound is:
A.
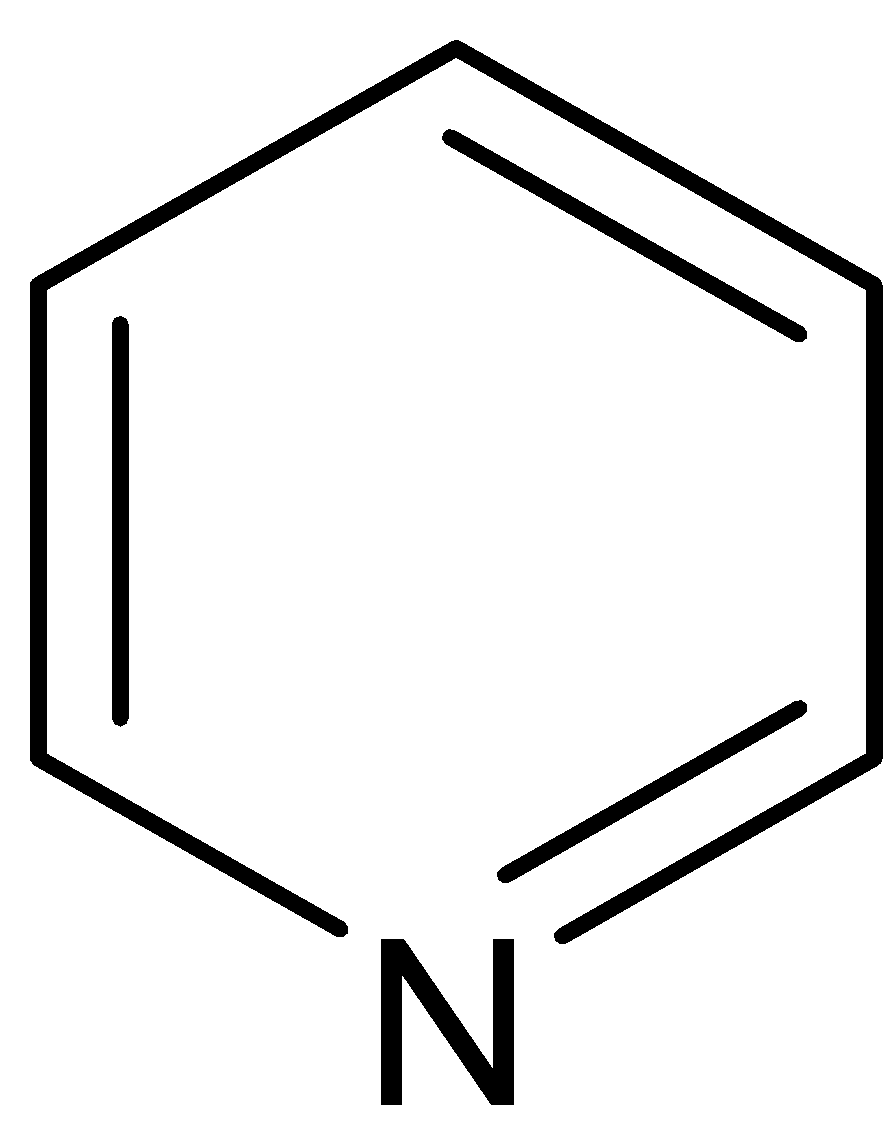
B.
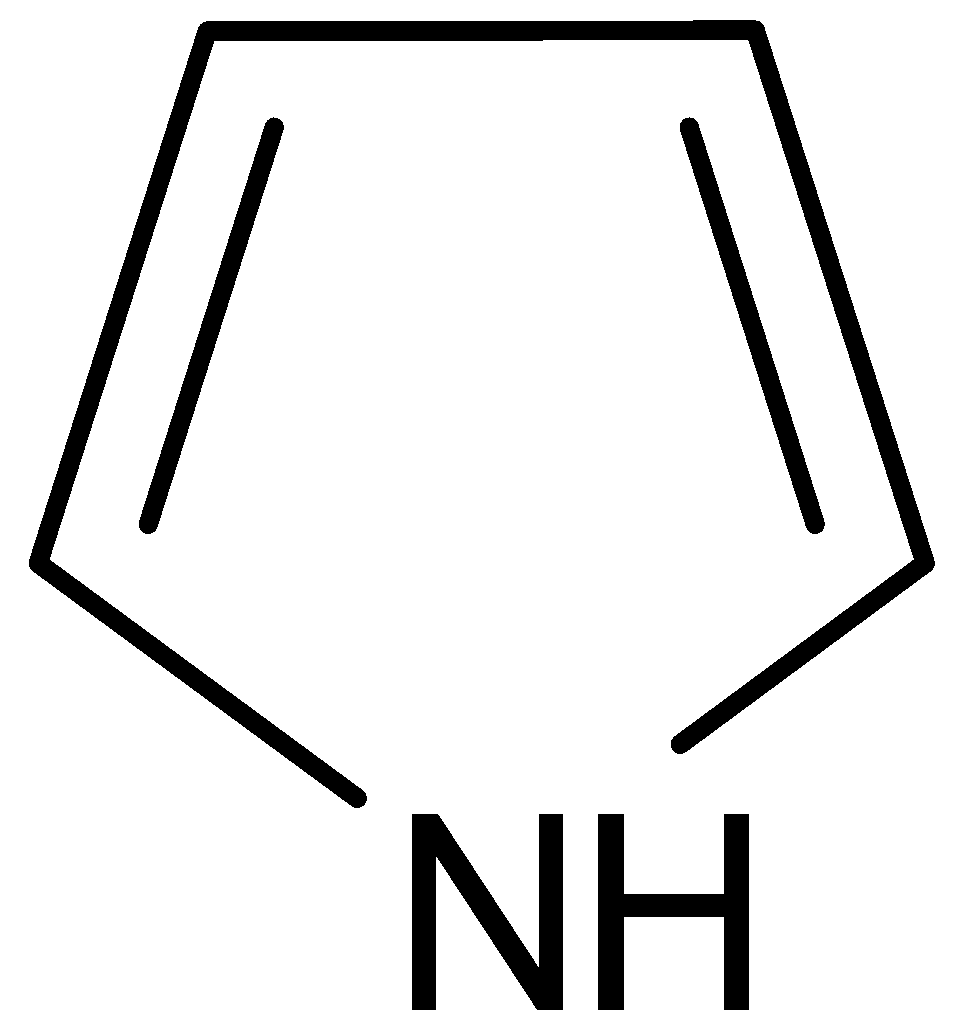
C.
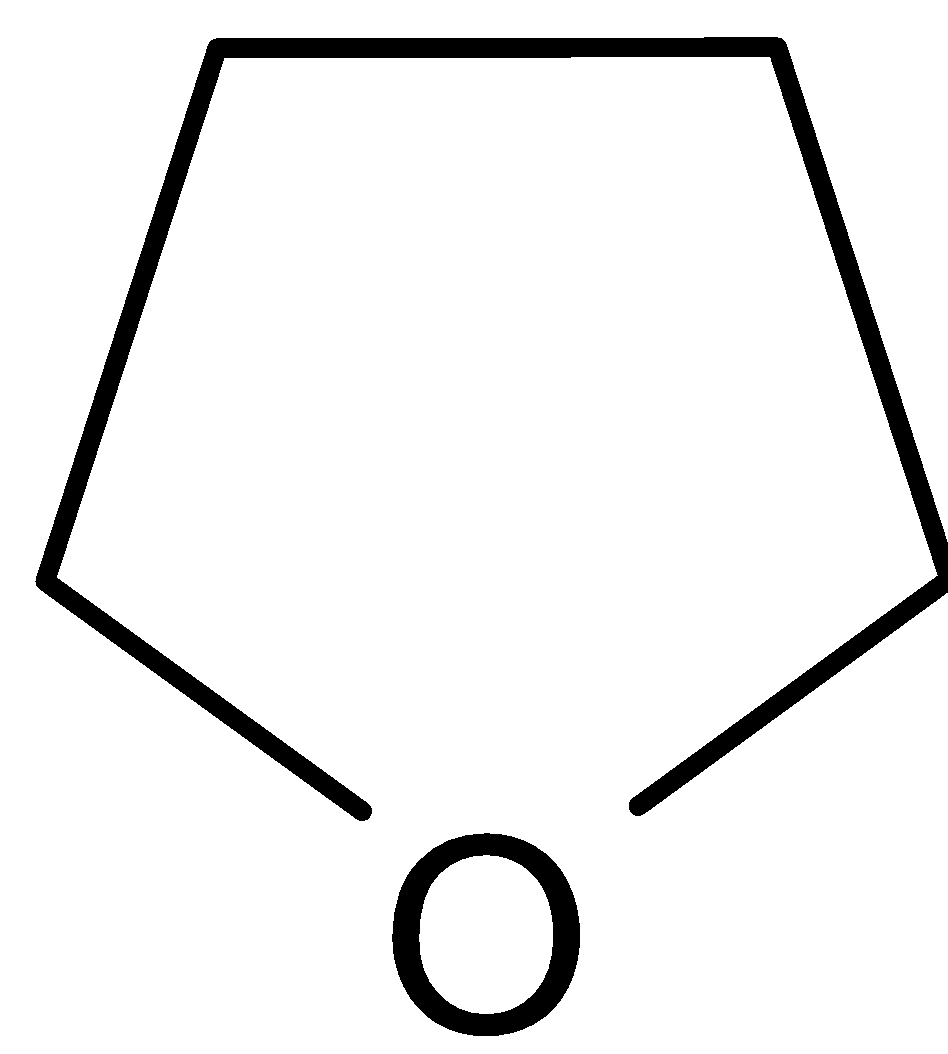
D.
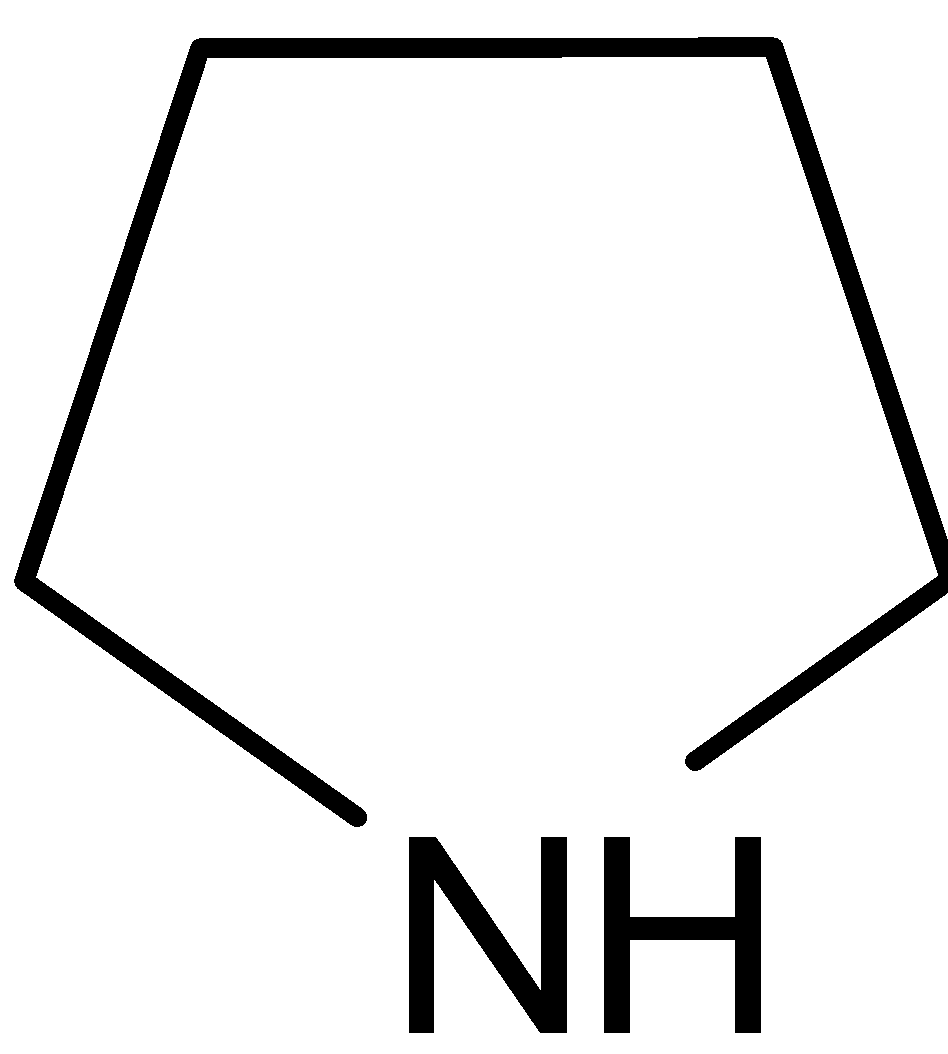




Answer
587.1k+ views
Hint: $sp_2$ lone pairs of electrons are more close to the nucleus than $sp_3$ lone pairs of electrons. Ability to donate electrons is the basis for basicity. When the lone pair of electrons take part in the formation of an aromatic six-electron \[\Pi \] cloud, it becomes difficult to donate or get protonated.
Complete step by step answer:
All the given options are heterocyclic compounds. They are rings composed of carbon and other atoms called heteroatoms.
A.
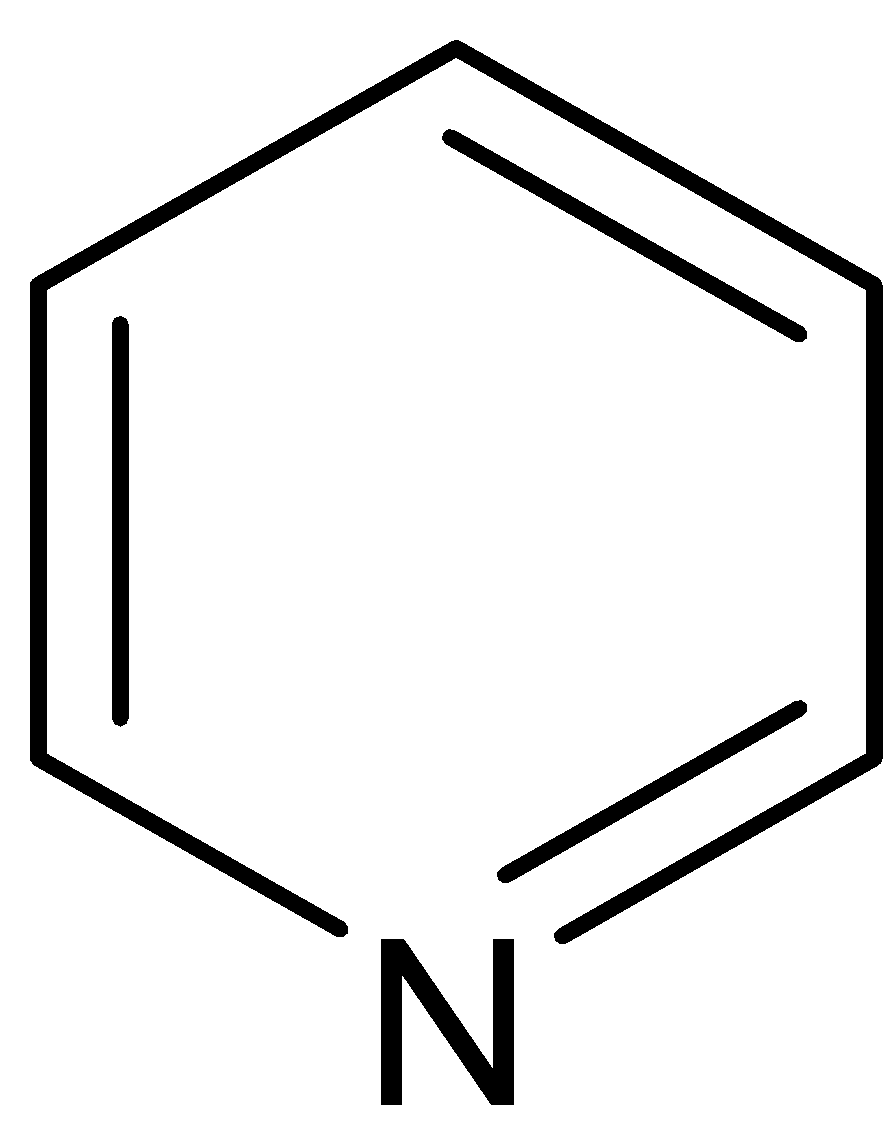
This compound is named as pyridine. It is a weak base because the electron pair in nitrogen is $sp_2$ hybridized. Moreover, the electron pair is very much tightly held by the atom. This lone pair of electrons can bind a proton by coordination to give pyridinium cation. Pyridine has 6 \[\Pi \] electrons distributed over 6 atoms. Thus it becomes electron deficient. Pyridine does not get involved in the aromatic sextet.
B.
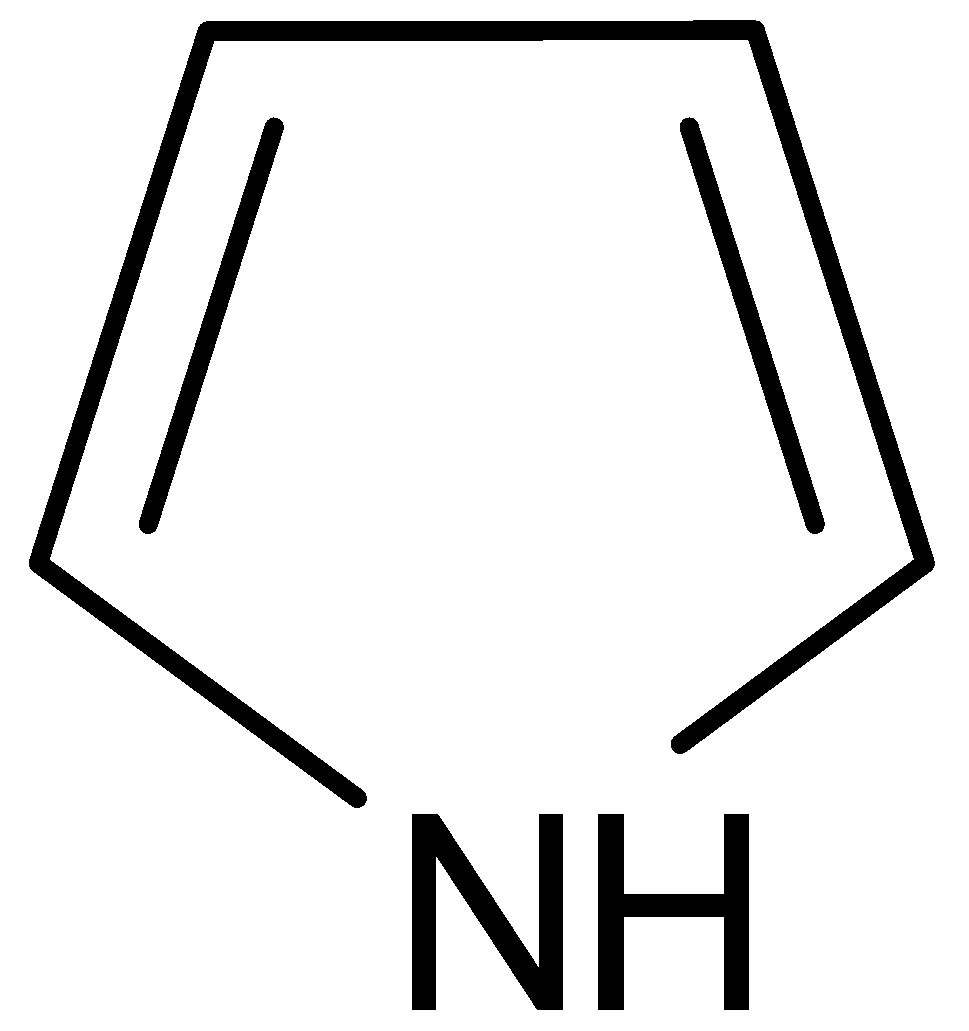
It is named as pyrrole. It has 3 pairs of delocalized \[\Pi \] electrons. Two of them are bonded and the third is lone pair on nitrogen. The lone pair of electrons in nitrogen is $sp_2$ hybridized. Pyrrole exhibits weak acidic and basic properties. It shows weak basic properties because the lone pair of electrons of nitrogen atom contributes to aromatic sextet.
C.
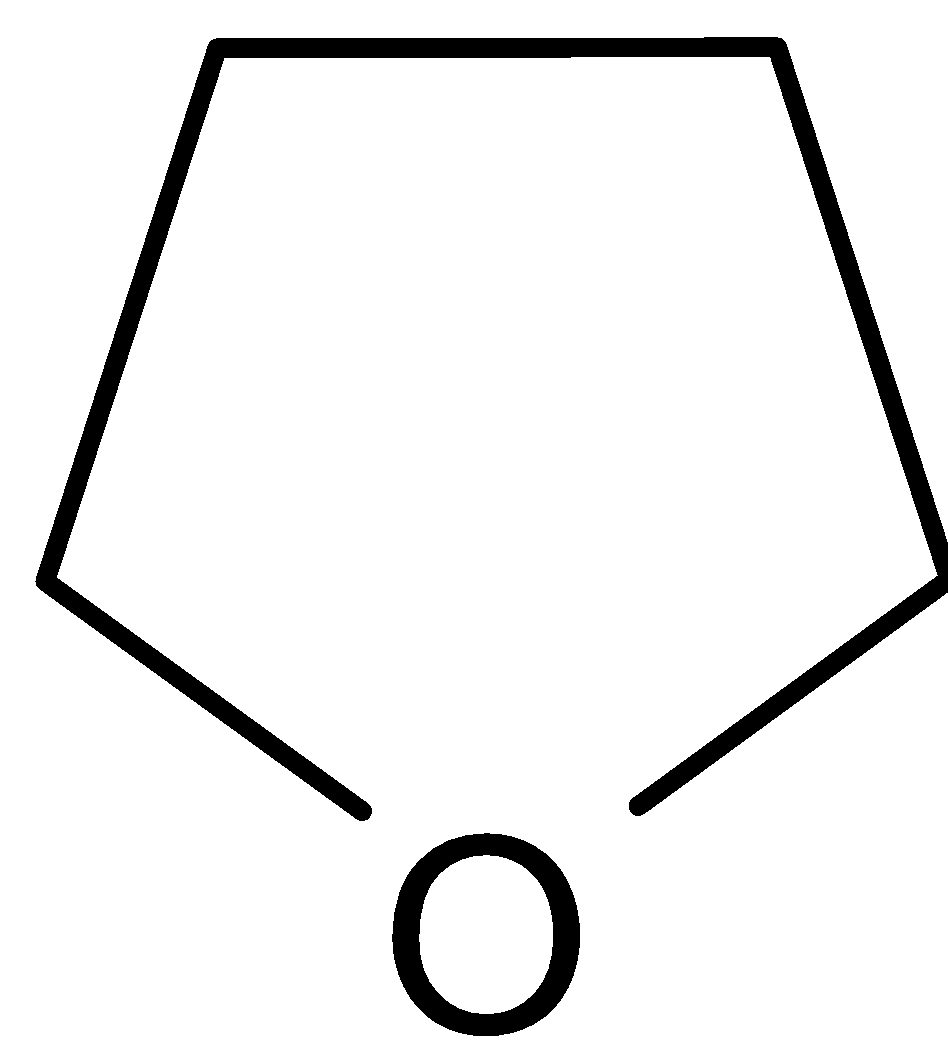
It is named as furan. It is less basic. It has a free $sp_2$ lone pair of electrons on nitrogen. So they can bind a proton. Still, it is more strongly bound to the nucleus than $sp_3$ lone pair.
D.
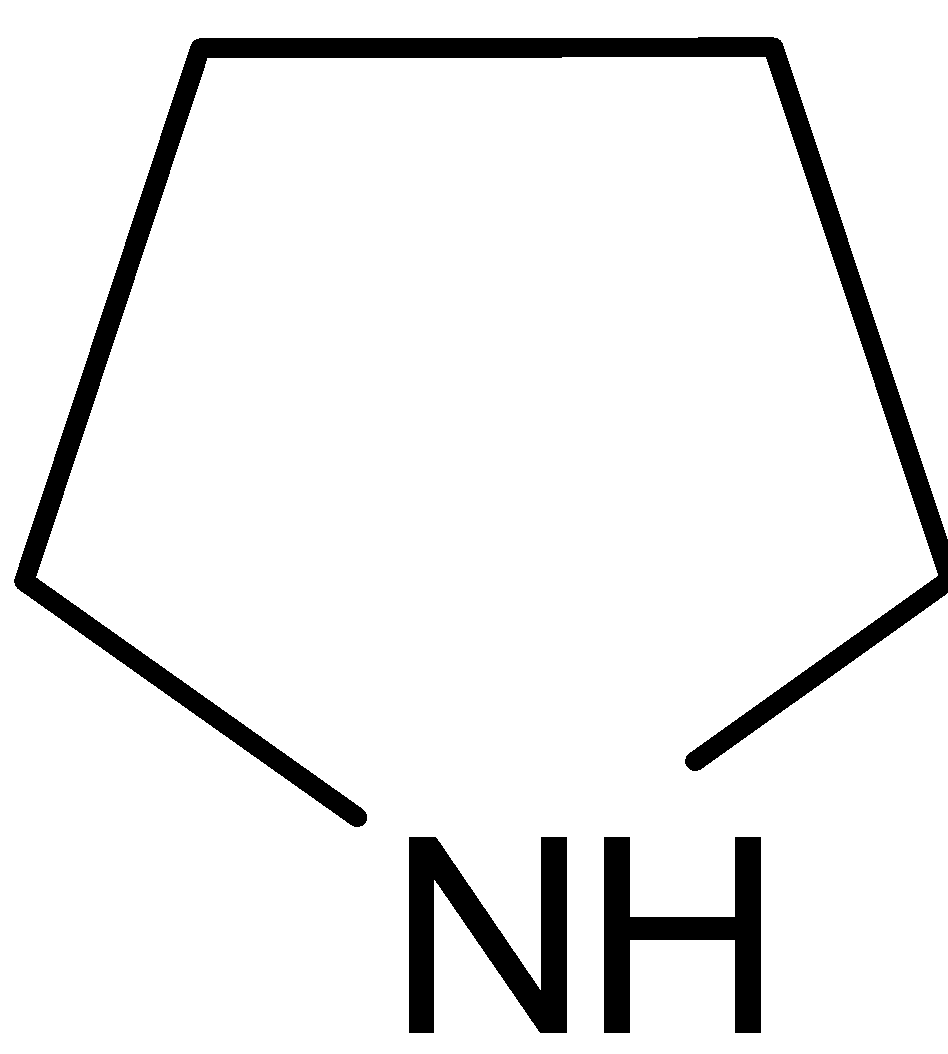
It is named as pyrrolidine. It is a cyclic secondary amine. Nitrogen is $sp_3$ hybridized. It does not have any double bonds. Nitrogen has a lone pair of electrons. The lone pair of electrons on nitrogen are more available so that it can be easily donated. Therefore it is a strong base.
Therefore option D is correct.
Additional information- Basicity of nitrogen containing compounds increases with increase in electron donating groups and decrease in electron withdrawing groups. Alkyl groups can be used as electron donating groups. Nitro groups can be used as electron withdrawing groups.
Note:
Nitrogen containing compounds are generally basic unless the unshared electron pair of nitrogen is conjugated with the \[\Pi \] electron pair. They can act as a Lewis base because its lone pair can form a bond with an electrophile.
Complete step by step answer:
All the given options are heterocyclic compounds. They are rings composed of carbon and other atoms called heteroatoms.
A.

This compound is named as pyridine. It is a weak base because the electron pair in nitrogen is $sp_2$ hybridized. Moreover, the electron pair is very much tightly held by the atom. This lone pair of electrons can bind a proton by coordination to give pyridinium cation. Pyridine has 6 \[\Pi \] electrons distributed over 6 atoms. Thus it becomes electron deficient. Pyridine does not get involved in the aromatic sextet.
B.

It is named as pyrrole. It has 3 pairs of delocalized \[\Pi \] electrons. Two of them are bonded and the third is lone pair on nitrogen. The lone pair of electrons in nitrogen is $sp_2$ hybridized. Pyrrole exhibits weak acidic and basic properties. It shows weak basic properties because the lone pair of electrons of nitrogen atom contributes to aromatic sextet.
C.

It is named as furan. It is less basic. It has a free $sp_2$ lone pair of electrons on nitrogen. So they can bind a proton. Still, it is more strongly bound to the nucleus than $sp_3$ lone pair.
D.

It is named as pyrrolidine. It is a cyclic secondary amine. Nitrogen is $sp_3$ hybridized. It does not have any double bonds. Nitrogen has a lone pair of electrons. The lone pair of electrons on nitrogen are more available so that it can be easily donated. Therefore it is a strong base.
Therefore option D is correct.
Additional information- Basicity of nitrogen containing compounds increases with increase in electron donating groups and decrease in electron withdrawing groups. Alkyl groups can be used as electron donating groups. Nitro groups can be used as electron withdrawing groups.
Note:
Nitrogen containing compounds are generally basic unless the unshared electron pair of nitrogen is conjugated with the \[\Pi \] electron pair. They can act as a Lewis base because its lone pair can form a bond with an electrophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




