
Among the following compounds, the increasing order of their basic strength is:
A. $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{IV}}} \right) < \left( {{\rm{III}}} \right)$
B. $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$
C. $\left( {\rm{I}} \right) < \left( {{\rm{II}}} \right) < \left( {{\rm{IV}}} \right) < \left( {{\rm{III}}} \right)$
D. $\left( {\rm{I}} \right) < \left( {{\rm{II}}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$

Answer
568.2k+ views
Hint: We know that the basic strength can be measured by accepting the hydrogen ion from the species. The compound which has greater ability to accept the hydrogen ions, also has the greater or higher basic strength.
Complete step-by-step answer:
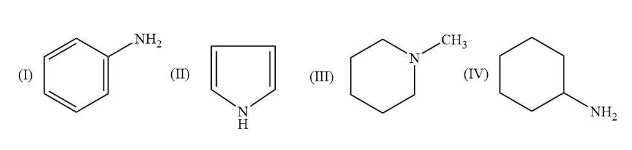
The compound II has least basic strength because the lone pairs of this compound are usually involved in the aromatization.
The compound I is less hindered and the lone pairs take part in the resonance.
The compound IV has highest basic strength because the nitrogen atom in this compound placed at ${\rm{s}}{{\rm{p}}^{\rm{3}}}$ hybridization and the size of each hydrogen atom is small so they are less bulky. In this compound, due to the alkyl group it shows +I effect and it is less hindered.
The compound III also shows +I effect due to alkyl group and it is also hindered.
So, the correct order for the increasing order of their basic strength is $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$.
Therefore, the correct option for this given question is B that is $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$.
Note: The organic compounds are usually containing carbon, hydrogen, nitrogen and oxygen as the main elements of the compound. They are used in the laboratory as a chemical reagent and also used in the synthesis of several other organic compounds.
Complete step-by-step answer:
The compound II has least basic strength because the lone pairs of this compound are usually involved in the aromatization.
The compound I is less hindered and the lone pairs take part in the resonance.
The compound IV has highest basic strength because the nitrogen atom in this compound placed at ${\rm{s}}{{\rm{p}}^{\rm{3}}}$ hybridization and the size of each hydrogen atom is small so they are less bulky. In this compound, due to the alkyl group it shows +I effect and it is less hindered.
The compound III also shows +I effect due to alkyl group and it is also hindered.
So, the correct order for the increasing order of their basic strength is $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$.
Therefore, the correct option for this given question is B that is $\left( {{\rm{II}}} \right) < \left( {\rm{I}} \right) < \left( {{\rm{III}}} \right) < \left( {{\rm{IV}}} \right)$.
Note: The organic compounds are usually containing carbon, hydrogen, nitrogen and oxygen as the main elements of the compound. They are used in the laboratory as a chemical reagent and also used in the synthesis of several other organic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




