
Why do amines behave as nucleophiles?
Answer
541.2k+ views
Hint: In organic chemistry, amines are referred to as the compounds or functional groups which are composed of a basic nitrogen atom having a lone pair of electrons. Amines are basically the derivatives of ammonia, in which one or more atoms of hydrogen have got replaced by a substituent like an alkyl or an aryl group.
Complete step by step answer:
A nucleophile refers to a compound which has the capacity to donate a pair of electrons to the electron deficient species. In simpler terms we can say that a nucleophile is anything which easily gets attracted towards, and attacks, either a positive or slightly positive portion of another ion or a molecule. And we know that all amines are composed of an active lone pair of electrons that is present on the very electronegative atom of nitrogen. This means that this lone pair of electrons are mainly attracted towards the positive parts of another ion or a molecule. In other words, we can say that Nitrogen in amines have the capacity to donate electrons owing to the presence of a lone pair of electrons. As a result, amines behave as nucleophiles.
For further clarification, let us look at the following cases:
(i) When lone pair of amine reacts with \[{H^ + }\], it acts as a base:
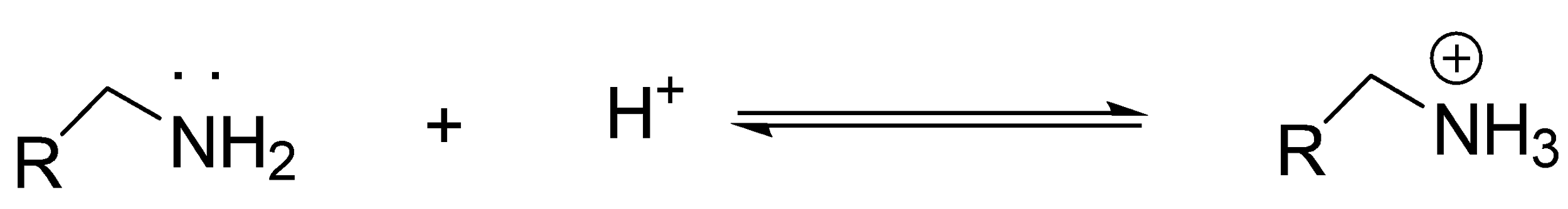
(ii) When lone pair of amine reacts with other atom instead of H, it acts as a nucleophile:
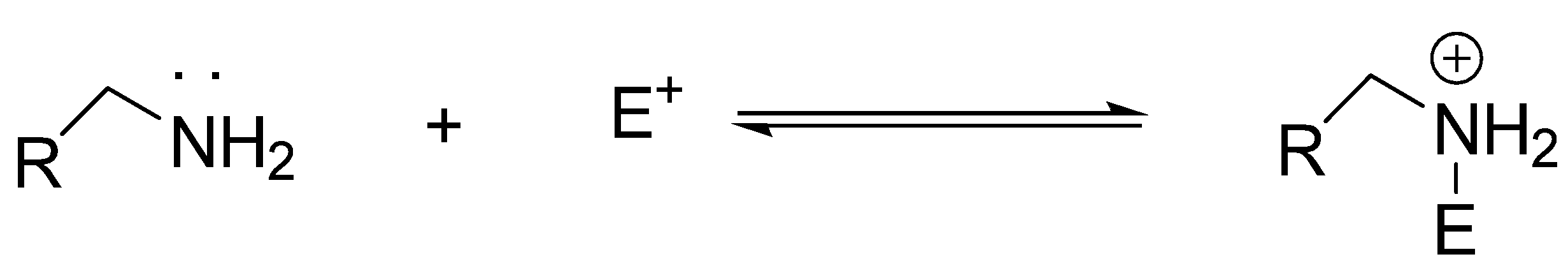
Note: Similarly, nucleophilic substitution reactions are those reactions in which an electron pair donor (i.e. a nucleophile say ‘Y’:) reacts with an electron pair acceptor (i.e. a substrate, say ‘R-X’) and which substitutes for the ‘X’ group (i.e. a leaving group). Let us look at the following generalized equation for nucleophilic substitution:
$Y{:^ - } + R - X \to Y - R + :{X^ - }$
Here, R can be an alkyl or an aryl group.
Complete step by step answer:
A nucleophile refers to a compound which has the capacity to donate a pair of electrons to the electron deficient species. In simpler terms we can say that a nucleophile is anything which easily gets attracted towards, and attacks, either a positive or slightly positive portion of another ion or a molecule. And we know that all amines are composed of an active lone pair of electrons that is present on the very electronegative atom of nitrogen. This means that this lone pair of electrons are mainly attracted towards the positive parts of another ion or a molecule. In other words, we can say that Nitrogen in amines have the capacity to donate electrons owing to the presence of a lone pair of electrons. As a result, amines behave as nucleophiles.
For further clarification, let us look at the following cases:
(i) When lone pair of amine reacts with \[{H^ + }\], it acts as a base:

(ii) When lone pair of amine reacts with other atom instead of H, it acts as a nucleophile:
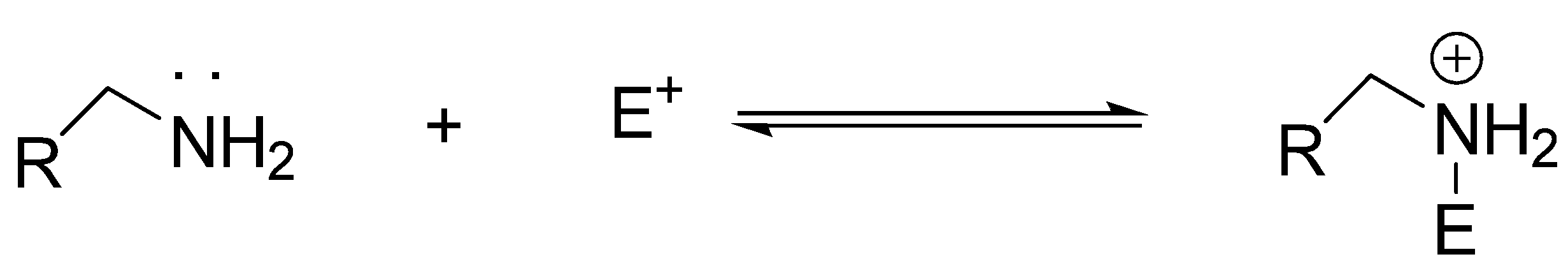
Note: Similarly, nucleophilic substitution reactions are those reactions in which an electron pair donor (i.e. a nucleophile say ‘Y’:) reacts with an electron pair acceptor (i.e. a substrate, say ‘R-X’) and which substitutes for the ‘X’ group (i.e. a leaving group). Let us look at the following generalized equation for nucleophilic substitution:
$Y{:^ - } + R - X \to Y - R + :{X^ - }$
Here, R can be an alkyl or an aryl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




