
Amines behave as:
A. Lewis acids
B. Lewis Base
C. Aprotic acid
D. neutral compound
Answer
578.4k+ views
Hint: Amines are derivatives of ammonia in which one or all three hydrogen atoms are replaced by alkyl groups.
\[N{H_3}\xrightarrow[{ - H}]{{ + R}}RN{H_2}\xrightarrow[{ - H}]{{ + R}}{R_2}NH\xrightarrow[{ - H}]{{ + R}}{R_3}N\]
\[1^\circ \]amine \[{2^\circ }\]amine \[{3^\circ }\]amine
Complete step by step answer:
We will explain basic nature of amine using primary amines \[ \leftarrow R - N{H_{^2}}\]
In amines, lone pairs of electrons are present on nitrogen atoms. They donate electrons during reaction and form bonds with other atoms.
The molecule which donates a lone pair of electrons is called Lewis base.
The molecule which accepts lone pairs of electrons is called Lewis acid.
\[\therefore \] Amines are Lewis Base
Hence option (B) is correct.
Additional information:
Basicity of amines increases from primary amines to tertiary amines.
Aliphatic amines are stronger bases than ammonia. Alkyl groups are electron releasing groups, it increases electron density on nitrogen atoms and therefore, they donate electrons easily.
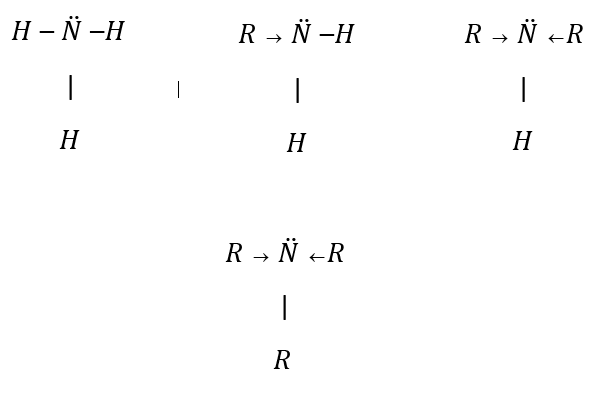
Number of alkyl groups increases from primary to tertiary amines. Alkyl group has a $ + I$ effect and it will increase electron density on nitrogen and therefore its basic nature increases. As a result its basic nature should be in order, Tertiary amine \[ > \] Secondary amine \[ > \] Primary amine.
This trend follows in a gaseous state. But in aqueous state basic character changes as,
Secondary amine \[ > \]Primary amine \[ > \] Tertiary amine
This is due to steric factors and solvation of ions. Aryl amines are less basic than alkyl amines due to delocalization of electron pairs in benzene rings.
Note:
Strength of Lewis base depends upon the availability of electron pairs present on nitrogen atoms. If it is easily available for bonding, then the compound is a strong base.
\[N{H_3}\xrightarrow[{ - H}]{{ + R}}RN{H_2}\xrightarrow[{ - H}]{{ + R}}{R_2}NH\xrightarrow[{ - H}]{{ + R}}{R_3}N\]
\[1^\circ \]amine \[{2^\circ }\]amine \[{3^\circ }\]amine
Complete step by step answer:
We will explain basic nature of amine using primary amines \[ \leftarrow R - N{H_{^2}}\]
In amines, lone pairs of electrons are present on nitrogen atoms. They donate electrons during reaction and form bonds with other atoms.
The molecule which donates a lone pair of electrons is called Lewis base.
The molecule which accepts lone pairs of electrons is called Lewis acid.
\[\therefore \] Amines are Lewis Base
Hence option (B) is correct.
Additional information:
Basicity of amines increases from primary amines to tertiary amines.
Aliphatic amines are stronger bases than ammonia. Alkyl groups are electron releasing groups, it increases electron density on nitrogen atoms and therefore, they donate electrons easily.
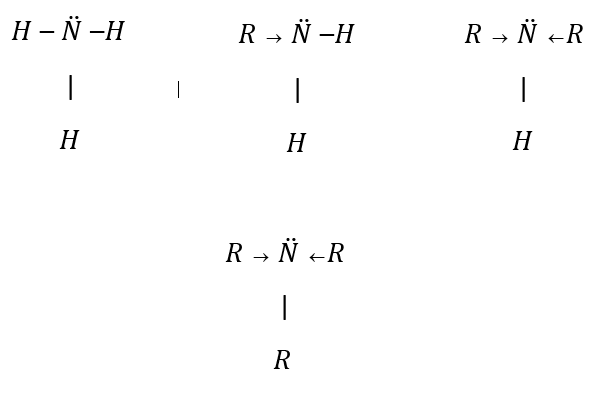
Number of alkyl groups increases from primary to tertiary amines. Alkyl group has a $ + I$ effect and it will increase electron density on nitrogen and therefore its basic nature increases. As a result its basic nature should be in order, Tertiary amine \[ > \] Secondary amine \[ > \] Primary amine.
This trend follows in a gaseous state. But in aqueous state basic character changes as,
Secondary amine \[ > \]Primary amine \[ > \] Tertiary amine
This is due to steric factors and solvation of ions. Aryl amines are less basic than alkyl amines due to delocalization of electron pairs in benzene rings.
Note:
Strength of Lewis base depends upon the availability of electron pairs present on nitrogen atoms. If it is easily available for bonding, then the compound is a strong base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




