
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. why?
Answer
599.4k+ views
Hint: Resonance is the way of describing the delocalization of bonded electrons or non-bonding electrons in a conjugated system. The intermediate structures formed are called resonating structure. The structure that collectively represents all resonating structures is believed to be most stable and is called the resonating hybrid.
Complete answer:
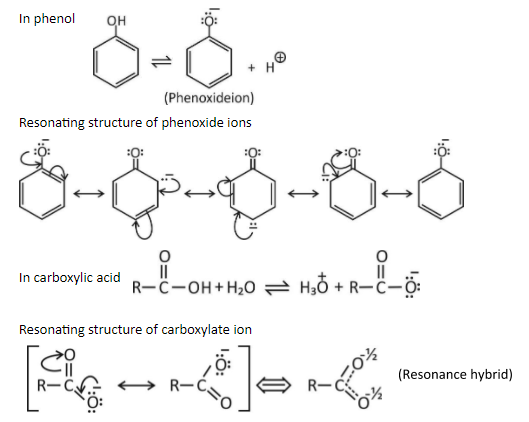
Carboxylic acid is more acidic than phenol. This can be explained on the basis of resonance, phenoxide ion has more number of resonating structures as compared to carboxylate ion.
In resonating structures of phenoxide ion, the negative charge is present on one electronegative oxygen atom and the less electronegative carbon atom. The contribution of resonance structures towards resonance stabilization of phenoxide ion is less.
In resonating structures of carboxylate ions, the negative charge is present on two electronegative oxygen atoms. The contribution of resonance structures towards resonance stabilization of carboxylate ion is more.
Hence carboxylate ions are more resonance stabilized than the phenoxide ions. Due to this the carboxylic acid is more acidic than phenol (higher the stability leads to greater acidic strength).
Note:Acidic strength of phenol and carboxylic acid depend upon stability of resonating structures. Carboxylate ions exhibit higher stability in comparison to phenoxide ions, thus higher stability leads to greater acidic strength.
Complete answer:

Carboxylic acid is more acidic than phenol. This can be explained on the basis of resonance, phenoxide ion has more number of resonating structures as compared to carboxylate ion.
In resonating structures of phenoxide ion, the negative charge is present on one electronegative oxygen atom and the less electronegative carbon atom. The contribution of resonance structures towards resonance stabilization of phenoxide ion is less.
In resonating structures of carboxylate ions, the negative charge is present on two electronegative oxygen atoms. The contribution of resonance structures towards resonance stabilization of carboxylate ion is more.
Hence carboxylate ions are more resonance stabilized than the phenoxide ions. Due to this the carboxylic acid is more acidic than phenol (higher the stability leads to greater acidic strength).
Note:Acidic strength of phenol and carboxylic acid depend upon stability of resonating structures. Carboxylate ions exhibit higher stability in comparison to phenoxide ions, thus higher stability leads to greater acidic strength.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




