
All the following may give meta-nitrophenol except:
A.
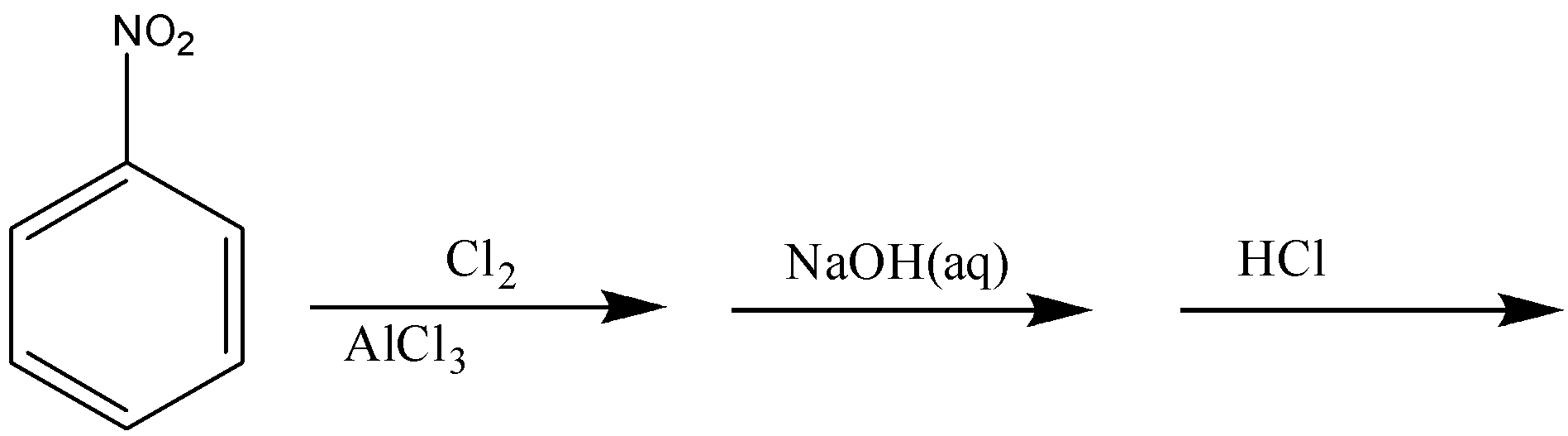
B.
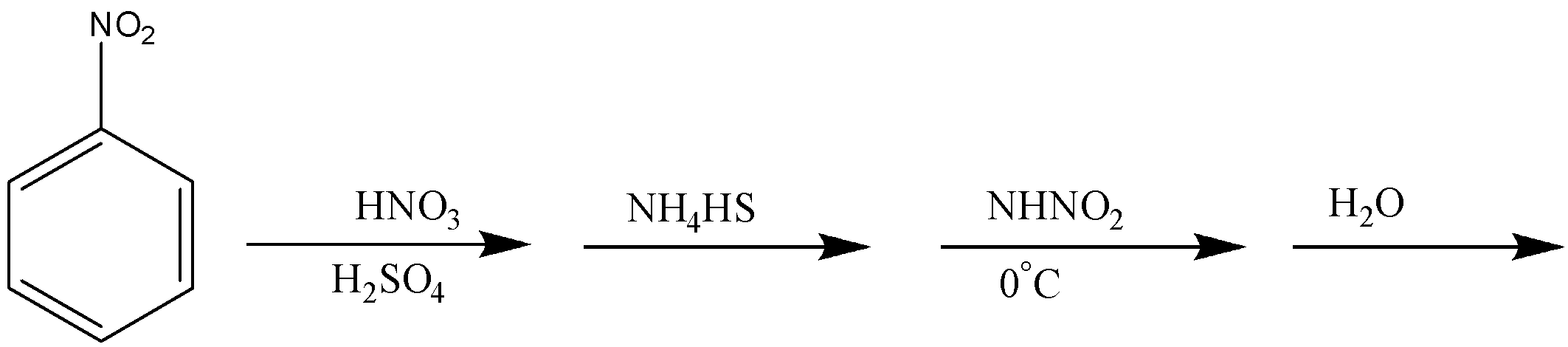
C.
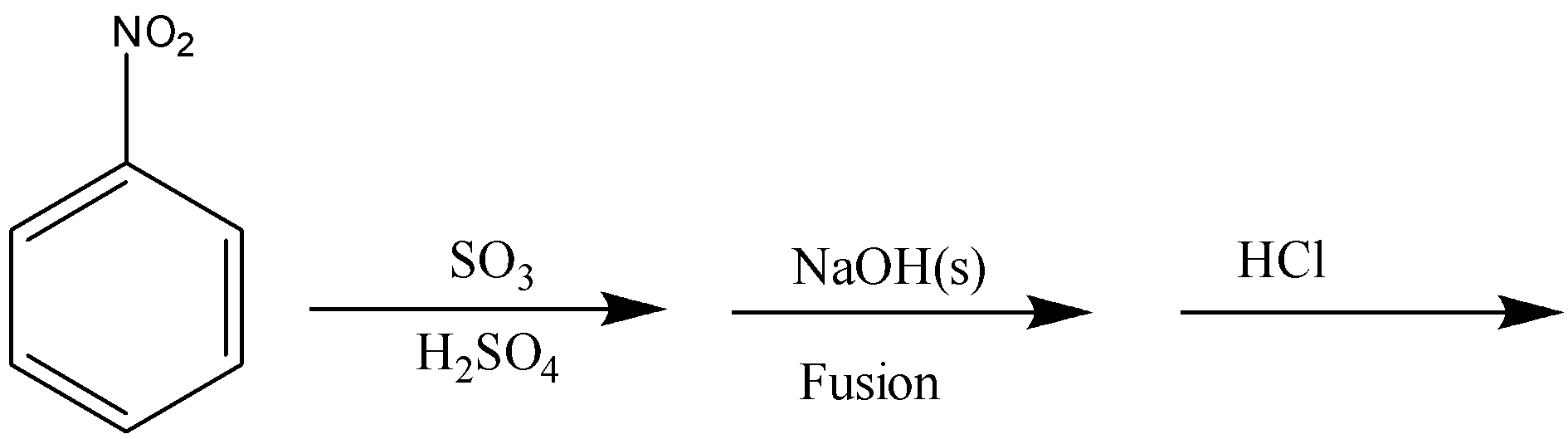
D.
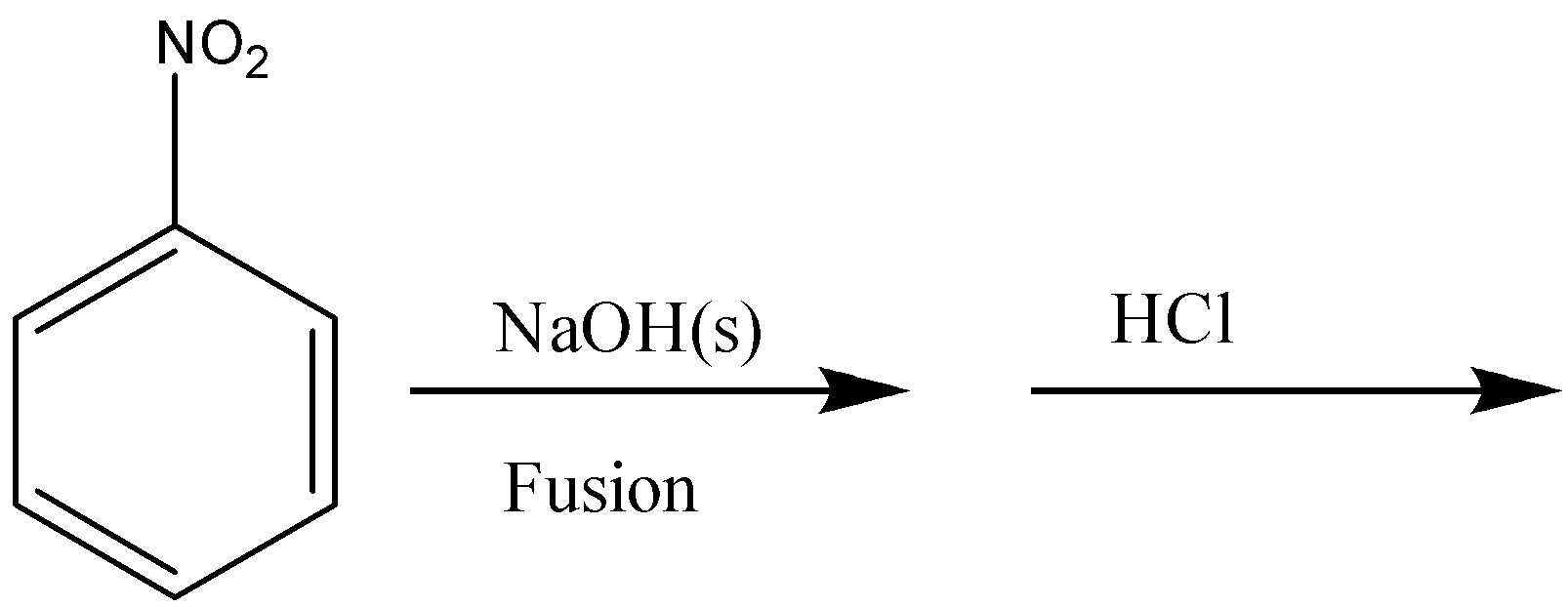




Answer
570k+ views
Hint: Nitrobenzene is an organic compound that is a pale-yellow oil with an almond-like odor. It is a water-insoluble compound. meta-nitrophenol is the hydroxyl derivative of nitrobenzene, in which a hydroxyl group is attached to meta position. Please note that nitro-group is a meta-directing group.
Complete step by step answer:
Nitro group is a ring deactivating group. It is not easy to replace hydrogen of nitrobenzene with the hydroxyl group, but if a better leaving group is attached to the ring, it is possible to substitute it with the hydroxyl group.
In the reaction given in option A, Cl will get attached to nitrobenzene at the meta-position, which then after reacting with NaOH will get replaced by ONa and will convert into OH after hydrolysis. Therefore, the reaction will give meta-nitrophenol as the final product.
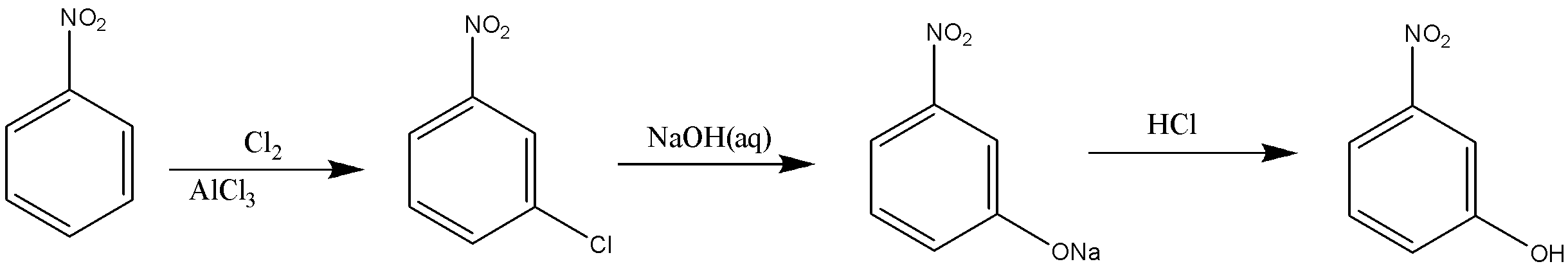
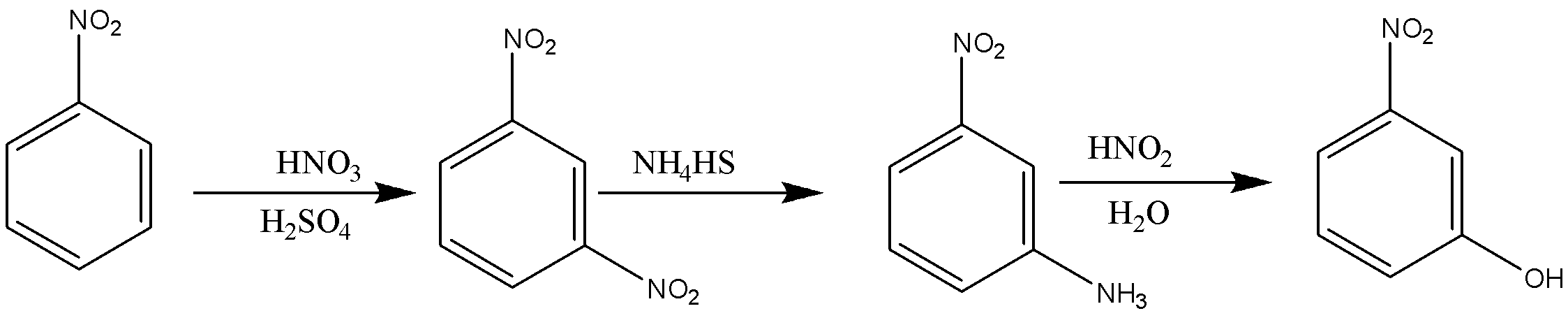
In the reaction given in option B, nitrobenzene on reaction with ${\text{HN}}{{\text{O}}_{\text{3}}}$ and ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ will form meta-dinitrobenzene, which will undergo partial hydrogenation in the presence of ${\text{N}}{{\text{H}}_{\text{4}}}{\text{HS}}$ and form meta-nitroaniline. This is the next step that will undergo diazotization and then in the presence of water will give meta-nitrophenol.
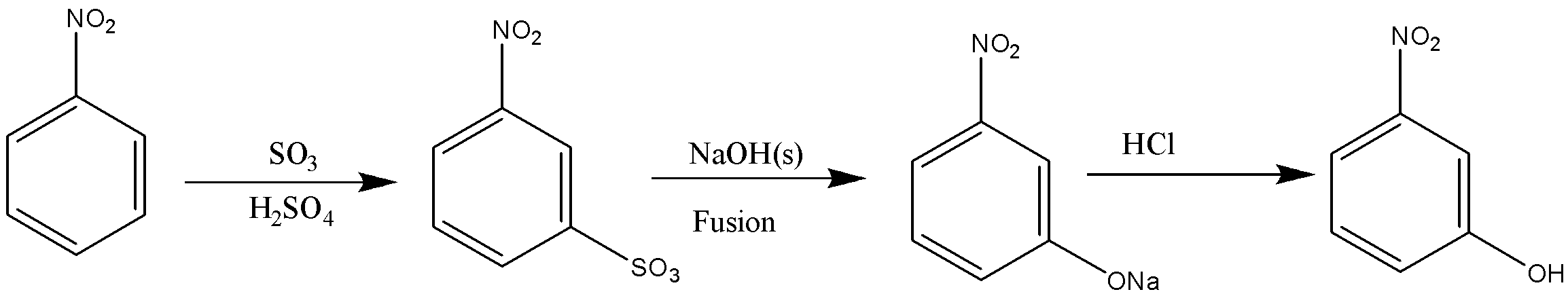
In the reaction given in option C, ${\text{S}}{{\text{O}}_{\text{3}}}$ will get attached to meta-position which the on fusion with NaOH will get replaced with ONa, and the in hydrolysis will give OH.
As stated earlier, nitrobenzene will not react with NaOH, therefore there will be no reaction in option D.
So, the correct answer is Option D .
Note: There are two types of substituents on the ring, one is meta-directing and the other one is ortho-para directing groups. The meta-directing groups are electron-withdrawing groups, they withdraw the electrons away from the ring and therefore, deactivate the ring. The ortho-para directing groups are electron-donating groups, these groups donate electron density to the ring, thus activating the ring.
Complete step by step answer:
Nitro group is a ring deactivating group. It is not easy to replace hydrogen of nitrobenzene with the hydroxyl group, but if a better leaving group is attached to the ring, it is possible to substitute it with the hydroxyl group.
In the reaction given in option A, Cl will get attached to nitrobenzene at the meta-position, which then after reacting with NaOH will get replaced by ONa and will convert into OH after hydrolysis. Therefore, the reaction will give meta-nitrophenol as the final product.

In the reaction given in option B, nitrobenzene on reaction with ${\text{HN}}{{\text{O}}_{\text{3}}}$ and ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ will form meta-dinitrobenzene, which will undergo partial hydrogenation in the presence of ${\text{N}}{{\text{H}}_{\text{4}}}{\text{HS}}$ and form meta-nitroaniline. This is the next step that will undergo diazotization and then in the presence of water will give meta-nitrophenol.

In the reaction given in option C, ${\text{S}}{{\text{O}}_{\text{3}}}$ will get attached to meta-position which the on fusion with NaOH will get replaced with ONa, and the in hydrolysis will give OH.

As stated earlier, nitrobenzene will not react with NaOH, therefore there will be no reaction in option D.
So, the correct answer is Option D .
Note: There are two types of substituents on the ring, one is meta-directing and the other one is ortho-para directing groups. The meta-directing groups are electron-withdrawing groups, they withdraw the electrons away from the ring and therefore, deactivate the ring. The ortho-para directing groups are electron-donating groups, these groups donate electron density to the ring, thus activating the ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




