
All the following IUPAC names are correct except:
(a) 1-chloro-1-methoxy propane
(b) 1-amino-1-ethoxypropane
(c) 1-Ethoxy-2-propanol
(d) 1- Ethoxy-1-propanamine
Answer
566.4k+ views
Hint: While writing the IUPAC name of any compound, we will first identify the primary group and the substituents attached to it and we will start numbering from that carbon from which the attached substituent is nearest and while writing the name that name of substituent will be written first which comes first in the alphabetic order along with the carbon number respectively. Now by using these rules of IUPAC naming , we can easily identify the incorrect IUPAC mane from the above given compounds.
Complete Solution :
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
While writing we must follow, the following certain rules as;
1. First , we have to identify the longest carbon chain and we have to number that in such a way the functional group attached to it gets the lowest number.
2. While writing the IUPAC naming of any compound, the suffix of the functional group is added at the end of the IUPAC name and if any substituents are attached to the compound then the prefix of those substituents are added at the starting of the carbon chain along with the carbon number to which they are attached.
So, we will write the IUPAC names of the above-mentioned compounds according to these rules.
now considering the statement:
(a) 1-chloro-1-methoxy propane
Its structure is as:
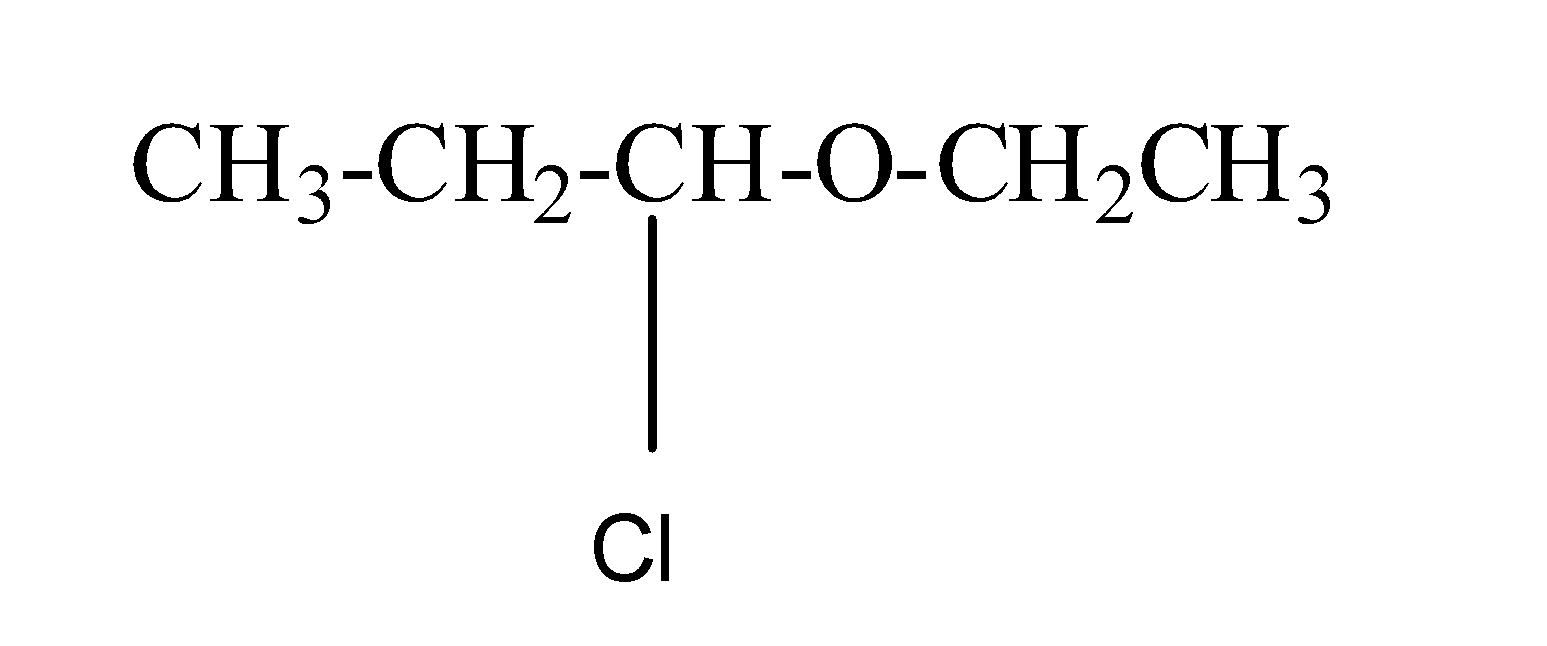
So, chlorine is present as a substituent group and ethoxy propane is the parent chain.
Thus, this IUPAC naming is correct.
(b) 1-amino-1-ethoxypropane
Its structure is as:
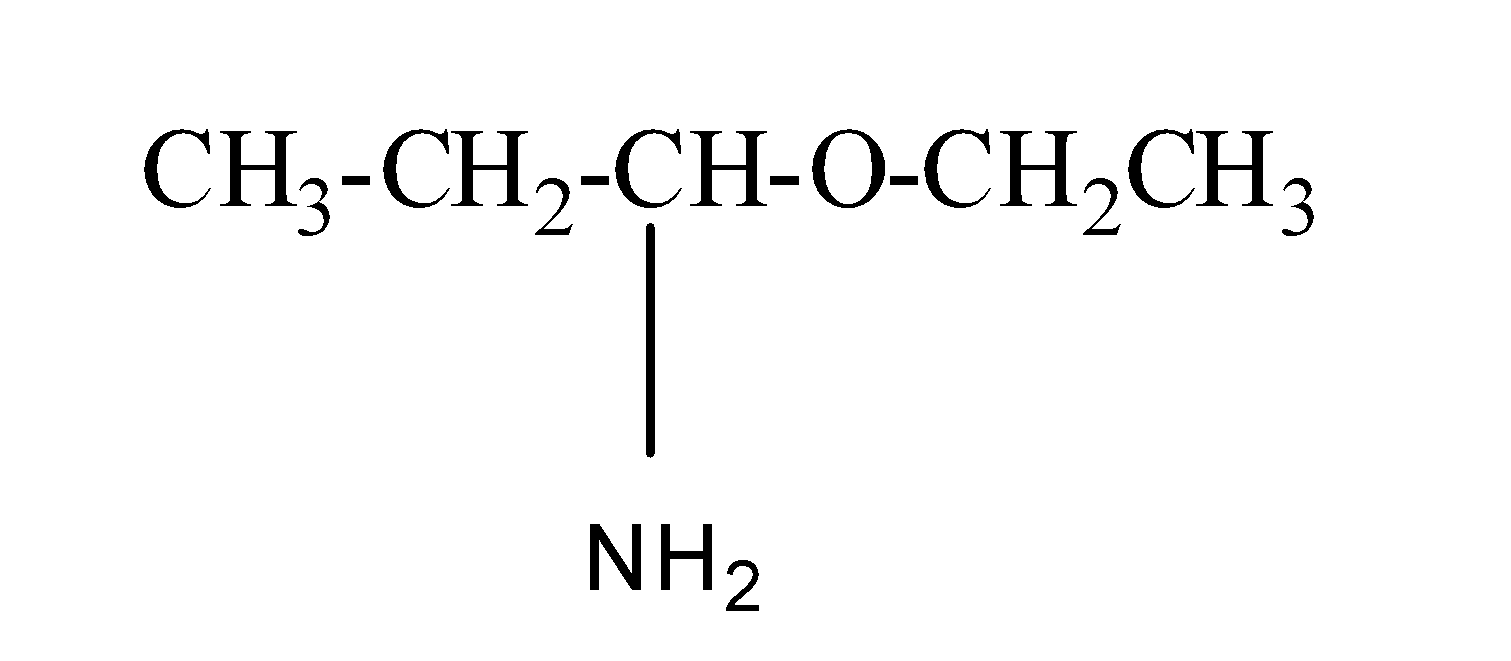
So, in this ethoxy is present as a substituent group and propan-1-amine is the parent chain.
Thus, this IUPAC naming is incorrect and the correct name is 1-ethoxypropan-1-amine.
(c) 1-Ethoxy-2-propanol
Its structure is as:
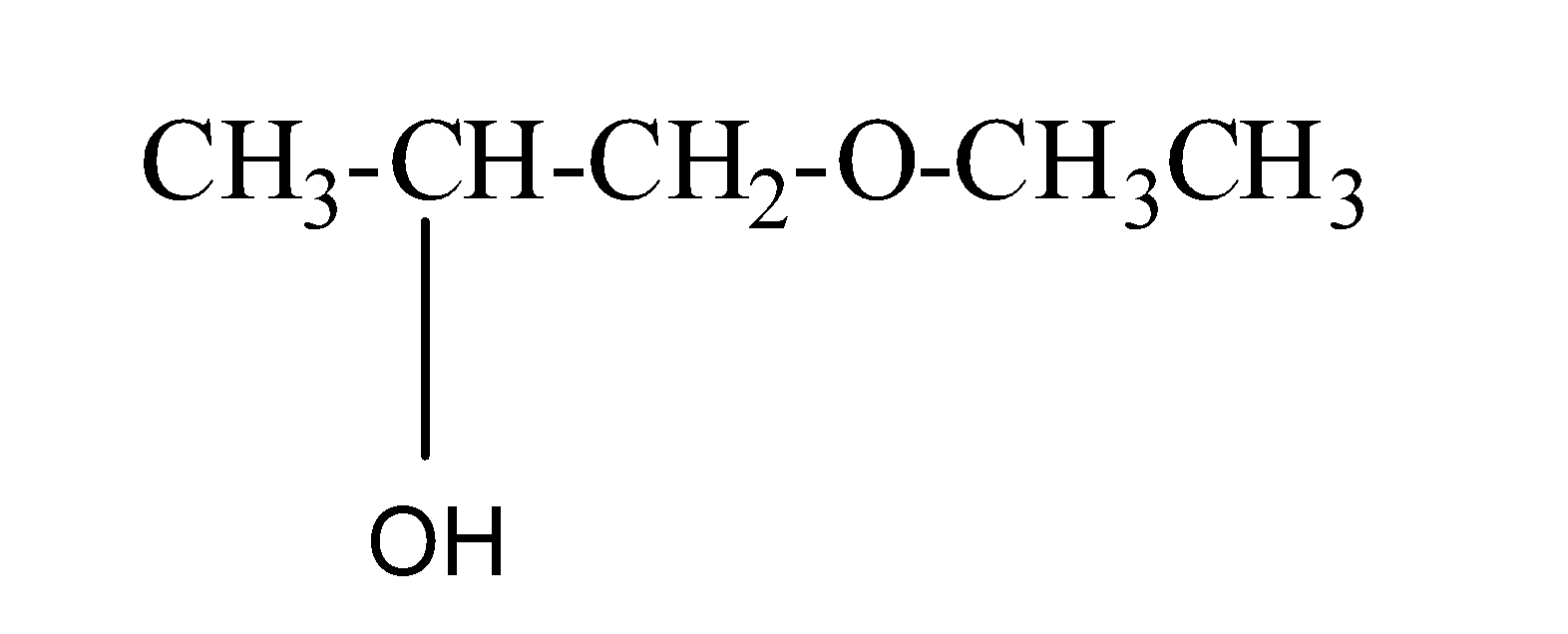
So, in this ethoxy is present as a substituent group and propan-2-ol is the parent chain.
Thus, this IUPAC naming is correct .
(d) 1- Ethoxy-1-propanamine
Its structure is as:
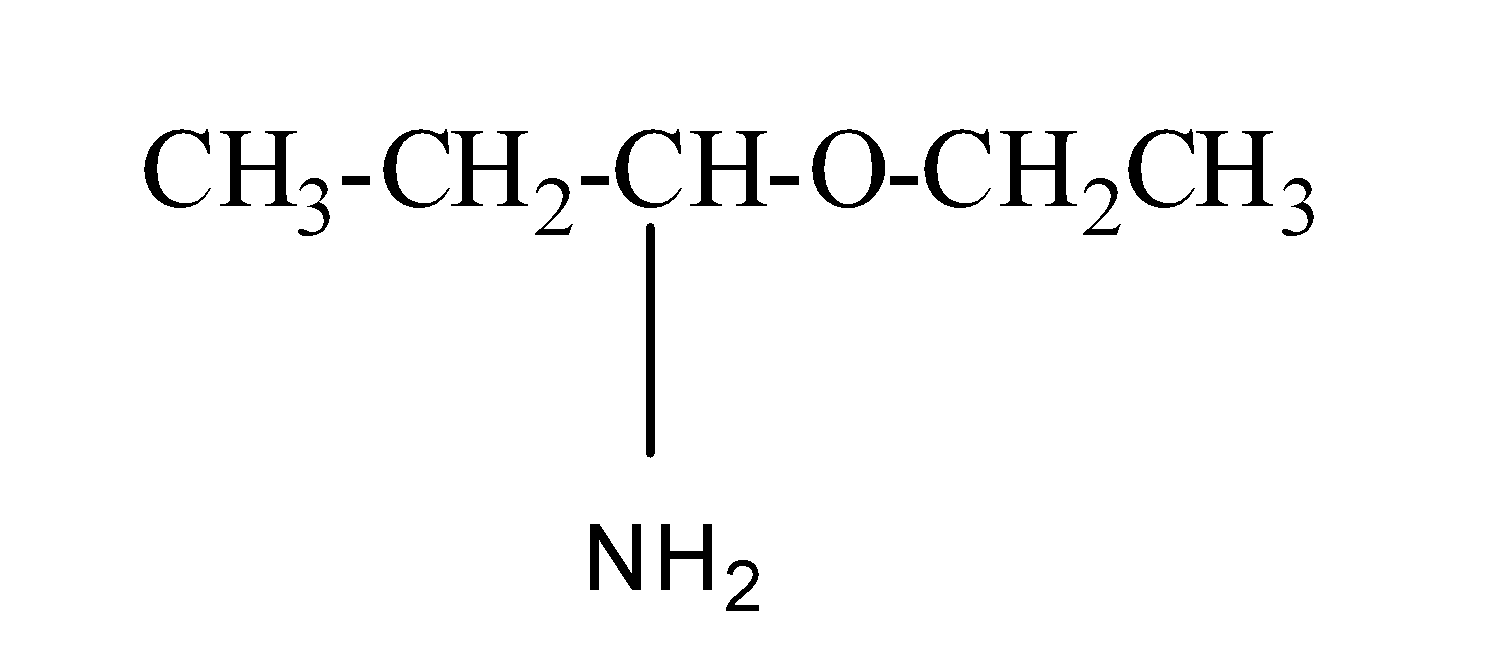
So, in this ethoxy is present as a substituent group and propan-1-amine is the parent chain.
Thus, this IUPAC naming is correct.
Hence, from the above , it is clear that all the IUPAC names are correct except 1-amino-1-ethoxypropane.
So, the correct answer is “Option B”.
Note: If there are two substituents attached to the compound, then we will write that name of the substituent first which comes first according to the alphabetic order thereafter followed by the next substituent and the compound name.
Complete Solution :
The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
While writing we must follow, the following certain rules as;
1. First , we have to identify the longest carbon chain and we have to number that in such a way the functional group attached to it gets the lowest number.
2. While writing the IUPAC naming of any compound, the suffix of the functional group is added at the end of the IUPAC name and if any substituents are attached to the compound then the prefix of those substituents are added at the starting of the carbon chain along with the carbon number to which they are attached.
So, we will write the IUPAC names of the above-mentioned compounds according to these rules.
now considering the statement:
(a) 1-chloro-1-methoxy propane
Its structure is as:

So, chlorine is present as a substituent group and ethoxy propane is the parent chain.
Thus, this IUPAC naming is correct.
(b) 1-amino-1-ethoxypropane
Its structure is as:

So, in this ethoxy is present as a substituent group and propan-1-amine is the parent chain.
Thus, this IUPAC naming is incorrect and the correct name is 1-ethoxypropan-1-amine.
(c) 1-Ethoxy-2-propanol
Its structure is as:
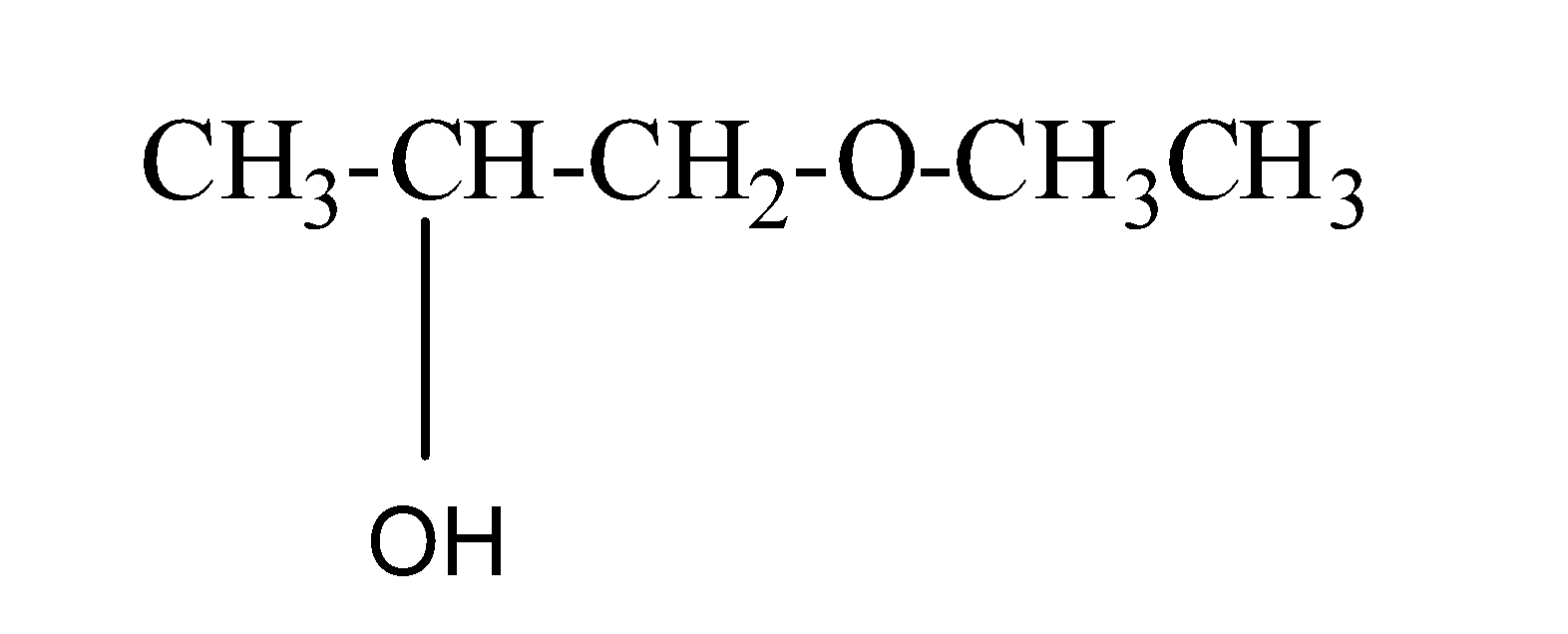
So, in this ethoxy is present as a substituent group and propan-2-ol is the parent chain.
Thus, this IUPAC naming is correct .
(d) 1- Ethoxy-1-propanamine
Its structure is as:

So, in this ethoxy is present as a substituent group and propan-1-amine is the parent chain.
Thus, this IUPAC naming is correct.
Hence, from the above , it is clear that all the IUPAC names are correct except 1-amino-1-ethoxypropane.
So, the correct answer is “Option B”.
Note: If there are two substituents attached to the compound, then we will write that name of the substituent first which comes first according to the alphabetic order thereafter followed by the next substituent and the compound name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




